PRF Lysates Modulate Chemokine Expression in Oral Squamous Carcinoma and Healthy Epithelial Cells
- PMID: 39199704
- PMCID: PMC11351820
- DOI: 10.3390/bioengineering11080746
PRF Lysates Modulate Chemokine Expression in Oral Squamous Carcinoma and Healthy Epithelial Cells
Abstract
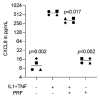
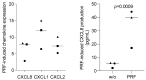
Platelet-rich fibrin (PRF), originally used to support soft tissue healing, is also considered a therapeutic option for treating oral lichen planus and leukoplakia. The progression from the two premalignant lesions to the aggressive malignant oral squamous cell carcinoma involves an inflammatory process linked to chemokine expression. Thus, there is a rationale for studying how PRF modulates the expression of chemokines in oral squamous carcinoma cells. To this aim, we expose the oral squamous carcinoma cell line HSC2 to IL1β and TNFα either alone or in the presence of lysates obtained from solid PRF membranes. We report here that in HSC2 cells, PRF lysates significantly reduce the forced transcription of chemokines, e.g., CXCL1, CXCL2, CXCL8, CXCL10, and CCL5. Moreover, PRF lysates attenuate the nuclear translocation of p65 in HSC2 oral epithelial cells when exposed to IL1β and TNFα. PRF lysates further reduce chemokine expression provoked by poly:IC HMW. Even though less pronounced, PRF lysates reduce IL1β- and TNFα-induced chemokine expression in TR146 cells. In primary oral epithelial cells, however, PRF lysates increase the basal expression of CXCL1, CXCL2 and CXCL8. Thus, PRF can exert a biphasic effect on chemokine expression in oral squamous cell carcinoma cell lines and primary oral epithelial cells. These findings suggest that PRF may reduce inflammation in a malignant environment while provoking an immunological response in healthy oral epithelium.
Keywords: HSC2; PRF; TR146; chemokines; inflammation; oral epithelial cells; oral squamous carcinoma cells; platelet-rich fibrin.
Conflict of interest statement
Salman Abbas Zadeh is an employee of epitome GmbH. Other authors declare no conflict of interest.
Figures


References
-
- Williams D.W., Greenwell-Wild T., Brenchley L., Dutzan N., Overmiller A., Sawaya A.P., Webb S., Martin D., Genomics N.N., Computational Biology C., et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184:4090–4104.e4015. doi: 10.1016/j.cell.2021.05.013. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

