Knockdown of Gonadotropin-Releasing Hormone II Receptor Impairs Ovulation Rate, Corpus Luteum Development, and Progesterone Production in Gilts
- PMID: 39199883
- PMCID: PMC11350859
- DOI: 10.3390/ani14162350
Knockdown of Gonadotropin-Releasing Hormone II Receptor Impairs Ovulation Rate, Corpus Luteum Development, and Progesterone Production in Gilts
Abstract
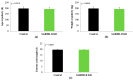
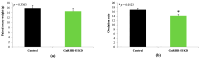
Reproduction is classically controlled by gonadotropin-releasing hormone (GnRH-I) and its receptor (GnRHR-I) within the brain. In pigs, a second form (GnRH-II) and its specific receptor (GnRHR-II) are also produced, with greater abundance in peripheral vs. central reproductive tissues. The binding of GnRH-II to GnRHR-II has been implicated in the autocrine/paracrine regulation of gonadal steroidogenesis rather than gonadotropin secretion. Blood samples were collected from transgenic gilts, with the ubiquitous knockdown of GnRHR-II (GnRHR-II KD; n = 8) and littermate controls (n = 7) at the onset of estrus (follicular) and 10 days later (luteal); serum concentrations of 16 steroid hormones were quantified by high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS). Upon euthanasia, ovarian weight (OWT), ovulation rate (OR), and the weight of each excised Corpus luteum (CLWT) were recorded; HPLC-MS/MS was performed on CL homogenates. During the luteal phase, serum progesterone concentration was reduced by 18% in GnRHR-II KD versus control gilts (p = 0.0329). Age and weight at puberty, estrous cycle length, and OWT were similar between lines (p > 0.05). Interestingly, OR was reduced (p = 0.0123), and total CLWT tended to be reduced (p = 0.0958) in GnRHR-II KD compared with control females. Luteal cells in CL sections from GnRHR-II KD gilts were hypotrophic (p < 0.0001). Therefore, GnRH-II and its receptor may help regulate OR, CL development, and progesterone production in gilts.
Keywords: Corpus luteum; GnRH-II; GnRH-II receptor; autocrine/paracrine regulation; gene knockdown; ovulation rate; porcine; progesterone; steroid hormone.
Conflict of interest statement
The authors declare no conflicts of interest. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The USDA prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program (not all prohibited bases apply to all programs.). Persons with disabilities who require alternative means for the communication of program information (braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, DC 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources

