Randomized, Double-Blind, Placebo-Controlled Study of Anti-Mycobacterial Therapy (RHB-104) in Active Crohn's Disease
- PMID: 39199994
- PMCID: PMC11350828
- DOI: 10.3390/antibiotics13080694
Randomized, Double-Blind, Placebo-Controlled Study of Anti-Mycobacterial Therapy (RHB-104) in Active Crohn's Disease
Abstract
This study, conducted between 4 October 2013, and 30 November 2018, tested the hypothesis that triple antimicrobial therapy, targeting Mycobacterium avium subspecies paratuberculosis (MAP), long considered a putative cause, would favorably affect Crohn's disease. A double-blind multicenter study of adults with active Crohn's disease, (i.e., Crohn's Disease Activity Index [CDAI] 220-450 plus C-reactive protein ≥ 1.0 mg/dL, fecal calprotectin (FCP) >162.9 µg/g stool, or recent endoscopic or radiographic confirmation of active disease) receiving concomitant standard-of-care Crohn's disease treatment (Clinicaltrials.gov: NCT01951326) were stratified by anti-tumor necrosis factor use and randomized (1:1) to anti-MAP RHB-104 (clarithromycin 95 mg, rifabutin 45 mg, and clofazimine 10 mg per capsule) (n = 166), resulting in clarithromycin 950 mg/day, rifabutin 450 mg/day, and clofazimine 100 mg/day, or placebo (n = 165) for up to 52 weeks. A greater proportion of RHB-104 versus placebo-treated patients met the primary endpoint-remission (i.e., CDAI < 150)-at week 26 (36.7% [61/166] vs. 22.4% [37/165], respectively; 95% CI for difference: 4.6, 24.0, p = 0.0048; chi-square test). Clinical response (reduction of CDAI by ≥100 points from baseline) at week 26 (first secondary endpoint) was also higher among the patients treated with RHB-104 (73/166 [44.0%]) compared with placebo (50/165 [30.3%]; 95% CI for difference: 3.4, 24.0, p = 0.0116), and it remained higher at week 52 among the patients treated with RHB-104 (59/166 [35.5%] vs. (35/165 [21.2%] for placebo; 95% CI for difference: 4.7, 23.9, p = 0.0042). A statistically significantly greater decline in FCP (another prospective efficacy endpoint) was also observed in RHB-104-treated patients, compared with placebo, at weeks 12, 26, and 52. The rates of serious adverse events were similar between groups (RHB-104: 18.7%; placebo: 18.8%). No patient died during the study. Antimicrobial therapy directed against MAP resulted in significantly greater improvement in clinical and laboratory (FCP) measures of active Crohn's disease.
Keywords: Crohn’s disease; Mycobacterium avium subspecies paratuberculosis; RHB-104; clarithromycin; clinical trial; clofazimine; rifabutin.
Conflict of interest statement
T.B. is on the Scientific Advisory Board of RedHill Biopharma, Ltd.; is a Medical Director/Consultant/Advisory Committee/Board member/Employee/Patent holder; has an ownership interest in the Centre for Digestive Diseases Pty Ltd.; is the Director Giaconda Ltd.; holds several patents related to enteric infections and inflammatory bowel disease; and holds company equity in Apple, Finch Th, Topelia Aust Ltd., and Tesla. D.Y.G. had expenses reimbursed from RedHill Biopharma Ltd. for presenting data at European and US conferences. D.Y.G., T.A., Z.H., S.L., H.S. and P.S. received research support from RedHill Biopharma Ltd. for their roles as investigators for the study reported herein (NCT01951326). In his laboratory at the University of Central Florida (UCF), S.A.N. processed and tested clinical samples, which were funded through a contract between RedHill Biopharma and UCF. S.A.N. is the inventor in a patent for the nested PCR and culture protocol used in the study reported herein. Redhill Biopharma has a licensing right to the patent via a licensing arrangement with UCF. A.B. was an employee of RedHill Biopharma at the time this study was conducted and holds company equity. R.F., C.F., P.M., P.A., M.S.H. and I.N.K. were consultants to RedHill Biopharma at the time this study was conducted and hold company equity. I.N.K. is a member of RedHill Biopharma’s Scientific Advisory Board. Author Scott Levenson was employed by Digestive Care Associates, Inc., Author Clara Fehrmann was employed by CEEF Solutions, Author M. Scott Harris was employed by Middleburg Consultants, Author Shuhong Zhao was employed by Syneos Health, Author Ira N. Kalfus was employed by M2g Consulting, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
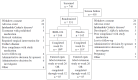


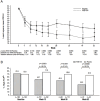
References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
-
- Peyrin-Biroulet L., Sandborn W., Sands B.E., Reinisch W., Bemelman W., Bryant R.V., D’Haens G., Dotan I., Dubinsky M., Feagan B., et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. - DOI - PubMed
-
- Kennedy N.A., Heap G.A., Green H.D., Hamilton B., Bewshea C., Walker G.J., Thomas A., Nice R., Perry M.H., Bouri S., et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol. Hepatol. 2019;4:341–353. doi: 10.1016/S2468-1253(19)30012-3. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

