Isavuconazole Pharmacokinetics in Critically Ill Patients: Relationship with Clinical Effectiveness and Patient Safety
- PMID: 39200006
- PMCID: PMC11350865
- DOI: 10.3390/antibiotics13080706
Isavuconazole Pharmacokinetics in Critically Ill Patients: Relationship with Clinical Effectiveness and Patient Safety
Abstract
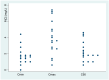
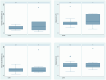
Isavuconazole is used to treat fungal infections. This study aims to describe isavuconazole pharmacokinetics in critically ill patients and evaluate their relationship with clinical efficacy and patient safety. We conducted a prospective, observational study in patients treated with intravenous isavuconazole. Samples were collected at predose (Cmin), 1 h (Cmax) and 12 h (C50) after the last dose. The plasma concentration was determined by high-performance liquid chromatography. The relationship between plasma concentration and clinical and microbiological outcomes and safety was evaluated. The influence of covariates (age, sex, weight, SAPS3, creatinine, liver enzymes and extracorporeal devices: continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO)) was analysed. Population pharmacokinetic modelling was performed using NONMEN®. A total of 71 isavuconazole samples from 24 patients were analysed. The mean Cmin was 1.76 (1.02) mg/L; 87.5% reached the optimal therapeutic target and 12.5% were below 1 mg/L. Population pharmacokinetics were best described by a one-compartment model with first-order elimination. No factor had a significant impact on the plasma concentration or pharmacokinetic parameters. Thus, isavuconazole could be safely used in a critically ill population, even in those treated with CRRT and ECMO, from a pharmacokinetic standpoint. Therefore, routine therapeutic drug monitoring may not be strictly necessary in daily clinical practice.
Keywords: antifungal; critical care; extracorporeal membrane oxygenation; isavuconazole; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Suberviola Cañas B., Jáuregui R., Ballesteros M.A., Leizaola O., González-Castro A., Castellanos-Ortega A. Efectos Del Retraso y La Inadecuación Del Tratamiento Antibiótico En La Supervivencia de Los Pacientes En Shock Séptico. Med. Intensiv. 2015;39:459–466. doi: 10.1016/j.medin.2014.12.006. - DOI - PubMed
-
- Abdul-Aziz M.H., Alffenaar J.W.C., Bassetti M., Bracht H., Dimopoulos G., Marriott D., Neely M.N., Paiva J.A., Pea F., Sjovall F., et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020;46:1127–1153. doi: 10.1007/s00134-020-06050-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous