Impact of Manual Addition of Vancomycin to Polymethylmethacrylate (PMMA) Cements
- PMID: 39200021
- PMCID: PMC11350699
- DOI: 10.3390/antibiotics13080721
Impact of Manual Addition of Vancomycin to Polymethylmethacrylate (PMMA) Cements
Abstract
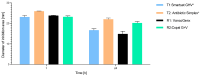
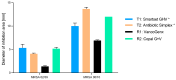
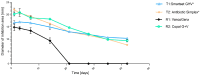
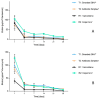
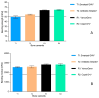
(1) Background: The addition of antibiotics to bone cements is a common practice in the treatment of periprosthetic joint infections. In revision cases, the amount and type of antibiotic is often insufficient and additional antibiotics must be added. The addition, however, changes the product itself, and the surgeon becomes the "manufacturer" of the bone cement. PMMAe wished to clarify whether the admixture of antibiotics changes the mechanical stability of the bone cements used and if the added antibiotics were still functional and released in sufficient quantities. (2) Methods: We compared two industrially manufactured vancomycin-containing PMMA cements; the low-viscous VancogenX® (TECRES, Sommacampagna, Italy) and the high-viscous Copal® G+V (Heraeus Medical GmbH, Wehrheim, Germany), with two PMMA cements loaded with aminoglycosides, to which 2.0 g of vancomycin (Hexal CT1631) were manually added-the high-viscous Smartset® GHV and the medium-viscous Antibiotic Simplex with Tobramycin (antibiotic Simplex® T). Test specimens of the bone cements were used to determine mechanical stability (bending strength and bending module), and the release of the antibiotics was determined by HLPC and modified Kirby-Bauer assays. (3) Results: All tested bone cements showed an initial high release within the first hours. Repeated testing after 24 h showed a reduced efficacy of VancogenX® and Smartset® GHV in Kirby-Bauer assays. Long-time release over days showed a release of functional antimicrobial active ingredients over this period of time in anti-microbial assays, but no activity of VancogenX® from day 21 onward. No significant differences in the ISO bending modules could be detected, but in contrast to the bending module, the ISO bending strength was substantially reduced by 10-15 mPal in comparison to both cements of the reference group. The Simplex®T met just the ISO 5833; the Smartset® GHV did not after adding vancomycin. (4) Conclusions: In conclusion, the manual addition of 2 g of vancomycin to 40 g of PMMA powder is recommended for the treatment of methicillin-resistant staphylococci. Vancomycin is released over a period of 42 days with concentrations above the MIC for typical staphylococci. The mechanical properties of the PMMA just met, or did not fulfill, ISO mechanical specification. Copal® G+V showed a better elution than VancogenX® over time.
Keywords: Copal G+V; PMMA; VancogenX; efficacy; elution; manually added vancomycin; mechanical properties.
Conflict of interest statement
The authors declare that this study received funding from Heraeus Medical GmbH. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures
References
-
- Kühn K.D. PMMA Cements Are We Aware What We Are Using? Springer; Berlin/Heidelberg, Germany: 2014. - DOI
-
- Chaiyakit P., Meknavin S., Honku N., Onklin I. Debridement, antibiotics, and implant retention combined with direct intra-articular antibiotic infusion in patients with acute hematogenous periprosthetic joint infection of the knee. BMC Musculoskelet. Disord. 2021;18:557. doi: 10.1186/s12891-021-04451-x. - DOI - PMC - PubMed
-
- Choe H., Maruo A., Hieda Y., Abe K., Kobayashi N., Ike H., Kumagai K., Takeyama M., Kawabata Y., Inaba Y. Novel local antibiotic antifungal treatment for fungal periprosthetic joint infection with continous local antibiotic perfusion: A surgical technique. Arthroplast. Today. 2023;24:101245. doi: 10.1016/j.artd.2023.101245. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

