Investigating the Antimicrobial Potential of 560 Compounds from the Pandemic Response Box and COVID Box against Resistant Gram-Negative Bacteria
- PMID: 39200023
- PMCID: PMC11350835
- DOI: 10.3390/antibiotics13080723
Investigating the Antimicrobial Potential of 560 Compounds from the Pandemic Response Box and COVID Box against Resistant Gram-Negative Bacteria
Abstract
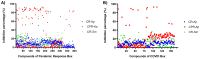
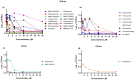
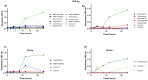
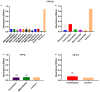
Antimicrobial resistance (AMR) has emerged as a significant threat to public health, particularly in infections caused by critically important Gram-negative bacteria. The development of novel antibiotics has its limitations, and therefore it is crucial to explore alternative strategies to effectively combat infections with resistant pathogens. In this context, the present study investigated the antibacterial potency of 560 compounds against the multidrug-resistant (MDR) strains of Klebsiella pneumoniae and Serratia marcescens. The evaluated compounds were selected from the Pandemic Response Box (PRB) and COVID Box (CB) and subjected to assays to determine the inhibitory concentration (IC), minimum bactericidal concentration (MBC), and biofilm formation. Further, the effects of these compounds on membrane integrity were assessed through protein quantification. Several of the evaluated compounds, including fusidic acid, MMV1580853, and MMV1634399, exhibited a significant reduction in biofilm formation and growth in K. pneumoniae. Trimethoprim exhibited potential against S. marcescens. The IC values of the compounds indicated significant microbial growth inhibition at various concentrations. These findings underscore the potency of the existing antibiotics and novel compounds in combating the MDR strains of bacteria. The importance of reconsidering the known antibiotics and utilizing drug repositioning strategies to address the increasing risk of AMR is highlighted.
Keywords: Gram-negative bacteria; antimicrobial resistance; drug screening; multidrug-resistant resistance.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Design SMAP29-LysPA26 as a Highly Efficient Artilysin against Pseudomonas aeruginosa with Bactericidal and Antibiofilm Activity.Microbiol Spectr. 2021 Dec 22;9(3):e0054621. doi: 10.1128/Spectrum.00546-21. Epub 2021 Dec 8. Microbiol Spectr. 2021. PMID: 34878337 Free PMC article.
-
Novel antimony-based antimicrobial drug targets membranes of Gram-positive and Gram-negative bacterial pathogens.Microbiol Spectr. 2024 Jun 4;12(6):e0423423. doi: 10.1128/spectrum.04234-23. Epub 2024 Apr 23. Microbiol Spectr. 2024. PMID: 38651882 Free PMC article.
-
Multidrug-Resistant Gram-Negative Bacteria and Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae from the Poultry Farm Environment.Microbiol Spectr. 2022 Jun 29;10(3):e0269421. doi: 10.1128/spectrum.02694-21. Epub 2022 Apr 25. Microbiol Spectr. 2022. PMID: 35467407 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
Cited by
-
Screening the Pandemic Response Box identifies novel ligands of the Staphylococcus aureus protein arginine kinase, McsB.Mol Biol Rep. 2025 May 6;52(1):446. doi: 10.1007/s11033-025-10545-9. Mol Biol Rep. 2025. PMID: 40327182 Free PMC article.
References
-
- Wise M.G., Karlowsky J.A., Mohamed N., Hermsen E.D., Kamat S., Townsend A., Brink A., Soriano A., Paterson D.L., Moore L.S.P., et al. Global trends in carbapenem- and difficult-to-treat-resistance among World Health Organization priority bacterial pathogens: ATLAS surveillance program 2018–2022. J. Glob. Antimicrob. Resist. 2024;37:168–175. doi: 10.1016/j.jgar.2024.03.020. - DOI - PubMed
-
- World Health Organization . Bacterial Priority Pathogens List, 2024. World Health Organization; Gevena, Switzerland: 2024.
-
- World Health Organization . Global Antimicrobial Resistance Surveillance System (GLASS) World Health Organization; Gevena, Switzerland: 2022. p. 80.
-
- Wei X.L., Zeng Q.L., Xie M., Bao Y. Pathogen Distribution, Drug Resistance Risk Factors, and Construction of Risk Prediction Model for Drug-Resistant Bacterial Infection in Hospitalized Patients at the Respiratory Department during the COVID-19 Pandemic. Infect. Drug Resist. 2023;16:1107–1121. doi: 10.2147/IDR.S399622. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

