Immunomodulatory Effects and Protection in Sepsis by the Antibiotic Moxifloxacin
- PMID: 39200042
- PMCID: PMC11350752
- DOI: 10.3390/antibiotics13080742
Immunomodulatory Effects and Protection in Sepsis by the Antibiotic Moxifloxacin
Abstract
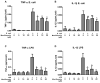
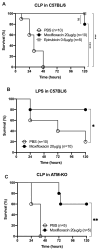
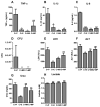
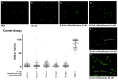
Sepsis is a leading cause of death in Intensive Care Units. Despite its prevalence, sepsis remains insufficiently understood, with no substantial qualitative improvements in its treatment in the past decades. Immunomodulatory agents may hold promise, given the significance of TNF-α and IL-1β as sepsis mediators. This study examines the immunomodulatory effects of moxifloxacin, a fluoroquinolone utilized in clinical practice. THP1 cells were treated in vitro with either PBS or moxifloxacin and subsequently challenged with lipopolysaccharide (LPS) or E. coli. C57BL/6 mice received intraperitoneal injections of LPS or underwent cecal ligation and puncture (CLP), followed by treatment with PBS, moxifloxacin, meropenem or epirubicin. Atm-/- mice underwent CLP and were treated with either PBS or moxifloxacin. Cytokine and organ lesion markers were quantified via ELISA, colony-forming units were assessed from mouse blood samples, and DNA damage was evaluated using a comet assay. Moxifloxacin inhibits the secretion of TNF-α and IL-1β in THP1 cells stimulated with LPS or E. coli. Intraperitoneal administration of moxifloxacin significantly increased the survival rate of mice with severe sepsis by 80% (p < 0.001), significantly reducing the plasma levels of cytokines and organ lesion markers. Notably, moxifloxacin exhibited no DNA damage in the comet assay, and Atm-/- mice were similarly protected following CLP, boasting an overall survival rate of 60% compared to their PBS-treated counterparts (p = 0.003). Moxifloxacin is an immunomodulatory agent, reducing TNF-α and IL-1β levels in immune cells stimulated with LPS and E. coli. Furthermore, moxifloxacin is also protective in an animal model of sepsis, leading to a significant reduction in cytokines and organ lesion markers. These effects appear unrelated to its antimicrobial activity or induction of DNA damage.
Keywords: IL-1β; TNF-α; antibiotics; moxifloxacin; quinolones; sepsis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337. - DOI - PubMed
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Fleischmann-Struzek C., Mellhammar L., Rose N., Cassini A., Rudd K.E., Schlattmann P., Allegranzi B., Reinhart K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46:1552–1562. doi: 10.1007/s00134-020-06151-x. - DOI - PMC - PubMed
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

