Association between Extended Meropenem Regimen and Achievement of Aggressive PK/PD in Patients Receiving Continuous Renal Replacement Therapy for Septic AKI
- PMID: 39200055
- PMCID: PMC11350760
- DOI: 10.3390/antibiotics13080755
Association between Extended Meropenem Regimen and Achievement of Aggressive PK/PD in Patients Receiving Continuous Renal Replacement Therapy for Septic AKI
Abstract
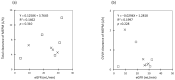
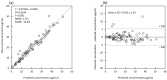
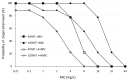
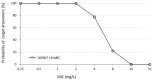
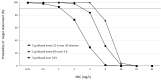
Aggressive pharmacokinetic (PK)/pharmacodynamic (PD) targets have shown better microbiological eradication rates and a lower propensity to develop resistant strains than conservative targets. We investigated whether meropenem blood levels, including aggressive PK/PD, were acceptable in terms of efficacy and safety using a meropenem regimen of 1 g infusion every 8 h over 3 h in patients undergoing continuous renal replacement therapy (CRRT) for septic acute kidney injury (AKI). Aggressive PK/PD targets were defined as the percentage of time that the free concentration (%fT) > 4 × minimal inhibitory concentration (MIC), the toxicity threshold was defined as a trough concentration >45 mg/L, and the percentage of achievement at each MIC was evaluated. The 100% fT > 4 × MIC for a pathogen with an MIC of 0.5 mg/L was 89%, and that for a pathogen with an MIC of 2 mg/L was 56%. The mean steady-state trough concentration of meropenem was 11.9 ± 9.0 mg/L and the maximum steady-state trough concentration was 29.2 mg/L. Simulations using Bayesian estimation showed the probability of achieving 100% fT > 4 × MIC for up to an MIC of 2 mg/L for the administered administration via continuous infusion at 3 g/24 h. We found that an aggressive PK/PD could be achieved up to an MIC of 0.5 mg/L with a meropenem regimen of 1 g infused every 8 h over 3 h for patients receiving CRRT for septic AKI. In addition, the risk of reaching the toxicity range with this regimen is low. In addition, if the MIC was 1-2 mg/L, the simulation results indicated that aggressive PK/PD can be achieved by continuous infusion at 3 g/24 h without increasing the daily dose.
Keywords: acute kidney injury; continuous renal replacement therapy; critically ill patients; meropenem; pharmacokinetics/pharmacodynamics analysis; therapeutic drug monitoring.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. - DOI - PMC - PubMed
-
- Garnacho-Montero J., Gutiérrez-Pizarraya A., Escoresca-Ortega A., Corcia-Palomo Y., Fernández-Delgado E., Herrera-Melero I., Ortiz-Leyba C., Márquez-Vácaro J.A. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40:32–40. doi: 10.1007/s00134-013-3077-7. - DOI - PubMed
-
- Abdul-Aziz M.H., Alffenaar J.C., Bassetti M., Bracht H., Dimopoulos G., Marriott D., Neely M.N., Paiva J.A., Pea F., Sjovall F., et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020;46:1127–1153. doi: 10.1007/s00134-020-06050-1. - DOI - PMC - PubMed
-
- Chua N.G., Loo L., Hee D.K.H., Lim T.P., Ng T.M., Hoo G.S.R., Soong J.L., Ong J.C.L., Tang S.S.L., Zhou Y.P., et al. Therapeutic drug monitoring of meropenem and piperacillin-tazobactam in the Singapore critically ill population—A prospective, multi-center, observational study (BLAST 1) J. Crit. Care. 2022;68:107–113. doi: 10.1016/j.jcrc.2021.12.013. - DOI - PubMed
-
- Gatti M., Cojutti P.G., Pascale R., Tonetti T., Laici C., Dell’Olio A., Siniscalchi A., Giannella M., Viale P., Pea F. Assessment of a PK/PD Target of Continuous Infusion beta-lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-negative Infections. Antibiotics. 2021;10:1311. doi: 10.3390/antibiotics10111311. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

