Synthesis of Second-Generation Analogs of Temporin-SHa Peptide Having Broad-Spectrum Antibacterial and Anticancer Effects
- PMID: 39200058
- PMCID: PMC11350846
- DOI: 10.3390/antibiotics13080758
Synthesis of Second-Generation Analogs of Temporin-SHa Peptide Having Broad-Spectrum Antibacterial and Anticancer Effects
Abstract
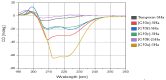
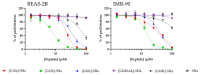
Antimicrobial peptides (AMPs) are a promising class of therapeutic alternatives with broad-spectrum activity against resistant pathogens. Small AMPs like temporin-SHa (1) and its first-generation analog [G10a]-SHa (2) possess notable efficacy against Gram-positive and Gram-negative bacteria. In an effort to further improve this antimicrobial activity, second-generation analogs of 1 were synthesised by replacing the natural glycine residue at position-10 of the parent molecule with atypical amino acids, such as D-Phenylalanine, D-Tyrosine and (2-Naphthyl)-D-alanine, to study the effect of hydrophobicity on antimicrobial efficacy. The resultant analogs (3-6) emerged as broad-spectrum antibacterial agents. Notably, the [G10K]-SHa analog (4), having a lysine substitution, demonstrated a 4-fold increase in activity against Gram-negative (Enterobacter cloacae DSM 30054) and Gram-positive (Enterococcus faecalis DSM 2570) bacteria relative to the parent peptide (1). Among all analogs, [G10f]-SHa peptide (3), featuring a D-Phe substitution, showed the most potent anticancer activity against lung cancer (A549), skin cancer (MNT-1), prostate cancer (PC-3), pancreatic cancer (MiaPaCa-2) and breast cancer (MCF-7) cells, achieving an IC50 value in the range of 3.6-6.8 µM; however, it was also found to be cytotoxic against normal cell lines as compared to [G10K]-SHa (4). Peptide 4 also possessed good anticancer activity but was found to be less cytotoxic against normal cell lines as compared to 1 and 3. These findings underscore the potential of second-generation temporin-SHa analogs, especially analog 4, as promising leads to develop new broad-spectrum antibacterial and anticancer agents.
Keywords: anticancer; antimicrobial peptide; temporin SHa.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Aamra H., Khan F.-A., Jahan H., Zafar M., Ali H., Shaheen F. Synthesis of novel benzimidazole containing antimicrobial peptides (AMPs) with significant inhibitory effect on multidrug resistant strain of Salmonella typhimurium. Synth. Commun. 2021;51:3620–3628. doi: 10.1080/00397911.2021.1986841. - DOI
-
- Khan F.-A., Yaqoob S., Qasim M.W., Ali S., Wang Y., Jiang Z.-H. A Robust, Gram-Scale and High-Yield Synthesis of MDP Congeners for Activation of the NOD2 Receptor and Vaccine Adjuvantation. Synthesis. 2024;56:539–548. doi: 10.1055/a-2004-5883. - DOI
-
- Khan F.A., Khanam R., Wasim Qasim M., Wang Y., Jiang Z.H.J.E. Improved Synthesis of D-Isoglutamine: Rapid Access to Desmuramyl Analogues of Muramyl Dipeptide for the Activation of Intracellular NOD2 Receptor and Vaccine Adjuvant Applications. Eur. J. Org. Chem. 2021;2021:6688–6699. doi: 10.1002/ejoc.202101170. - DOI
-
- Giuliani A., Pirri G., Nicoletto S. Antimicrobial peptides: An overview of a promising class of therapeutics. Open Life Sci. 2007;2:1–33. doi: 10.2478/s11535-007-0010-5. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

