An Insight into Rational Drug Design: The Development of In-House Azole Compounds with Antimicrobial Activity
- PMID: 39200063
- PMCID: PMC11350776
- DOI: 10.3390/antibiotics13080763
An Insight into Rational Drug Design: The Development of In-House Azole Compounds with Antimicrobial Activity
Abstract
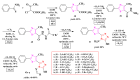
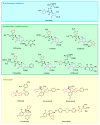
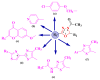
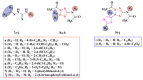
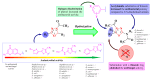
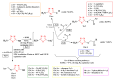
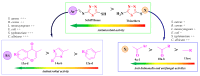
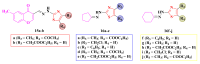
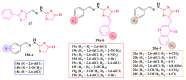
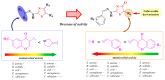
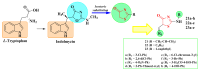
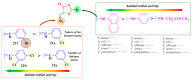
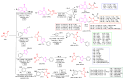
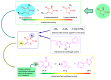
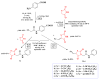
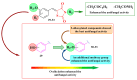
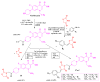
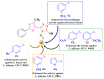
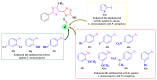
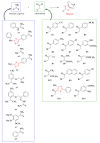
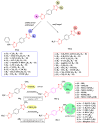
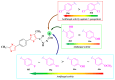
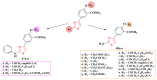
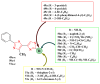
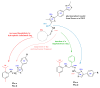
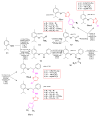
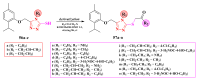
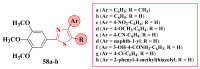
Antimicrobial resistance poses a major threat to global health as the number of efficient antimicrobials decreases and the number of resistant pathogens rises. Our research group has been actively involved in the design of novel antimicrobial drugs. The blueprints of these compounds were azolic heterocycles, particularly thiazole. Starting with oxadiazolines, our research group explored, one by one, the other five-membered heterocycles, developing more or less potent compounds. An overview of this research activity conducted by our research group allowed us to observe an evolution in the methodology used (from inhibition zone diameters to minimal inhibitory concentrations and antibiofilm potential determination) correlated with the design of azole compounds based on results obtained from molecular modeling. The purpose of this review is to present the development of in-house azole compounds with antimicrobial activity, designed over the years by this research group from the departments of Pharmaceutical and Therapeutical Chemistry in Cluj-Napoca.
Keywords: antimicrobial; azoles; heterocycles; organic synthesis; structure-activity relationship.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
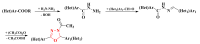
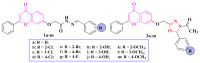
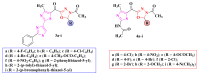
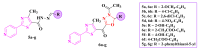
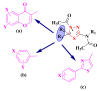
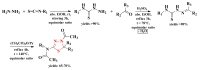
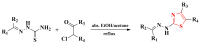
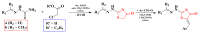
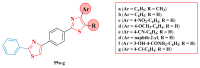
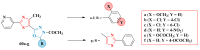
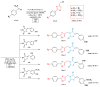
References
-
- Allel K., Day L., Hamilton A., Lin L., Furuya-Kanamori L., Moore C.E., Van Boeckel T., Laxminarayan R., Yakob L. Global Antimicrobial-Resistance Drivers: An Ecological Country-Level Study at the Human–Animal Interface. Lancet Planet. Health. 2023;7:e291–e303. doi: 10.1016/S2542-5196(23)00026-8. - DOI - PubMed
-
- WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022. 2022. [(accessed on 2 August 2024)]. Available online: https://www.who.int/publications/i/item/9789240062702.
-
- WHO Antimicrobial Resistance. 2023. [(accessed on 2 August 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

