Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces
- PMID: 39200087
- PMCID: PMC11351874
- DOI: 10.3390/antibiotics13080788
Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces
Abstract
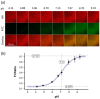
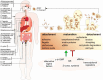
The growing threat of antimicrobial-resistant (AMR) pathogens to human health worldwide emphasizes the need for more effective infection control strategies. Bacterial and fungal biofilms pose a major challenge in treating AMR pathogen infections. Biofilms are formed by pathogenic microbes encased in extracellular polymeric substances to confer protection from antimicrobials and the host immune system. Biofilms also promote the growth of antibiotic-resistant mutants and latent persister cells and thus complicate therapeutic approaches. Biofilms are ubiquitous and cause serious health risks due to their ability to colonize various surfaces, including human tissues, medical devices, and food-processing equipment. Detection and characterization of biofilms are crucial for prompt intervention and infection control. To this end, traditional approaches are often effective, yet they fail to identify the microbial species inside biofilms. Recent advances in artificial intelligence (AI) have provided new avenues to improve biofilm identification. Machine-learning algorithms and image-processing techniques have shown promise for the accurate and efficient detection of biofilm-forming microorganisms on biotic and abiotic surfaces. These advancements have the potential to transform biofilm research and clinical practice by allowing faster diagnosis and more tailored therapy. This comprehensive review focuses on the application of AI techniques for the identification of biofilm-forming pathogens in various industries, including healthcare, food safety, and agriculture. The review discusses the existing approaches, challenges, and potential applications of AI in biofilm research, with a particular focus on the role of AI in improving diagnostic capacities and guiding preventative actions. The synthesis of the current knowledge and future directions, as described in this review, will guide future research and development efforts in combating biofilm-associated infections.
Keywords: abiotic surfaces; antimicrobial resistance; artificial intelligence; biofilm formation; biotic surfaces; deep learning; image processing; machine learning; microbial pathogens.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
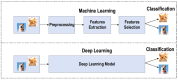
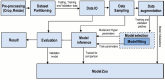


References
-
- Nabadda S., Kakooza F., Kiggundu R., Walwema R., Bazira J., Mayito J., Mugerwa I., Sekamatte M., Kambugu A., Lamorde M. Implementation of the World Health Organization global antimicrobial resistance surveillance system in Uganda, 2015–2020: Mixed-methods study using national surveillance data. JMIR Public Health Surveill. 2021;7:e29954. doi: 10.2196/29954. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services, Centres for Disease Control and Prevention; Atlanta, GA, USA: 2019.
-
- ECDC. EMEA . The Bacterial Challenge–Time to React a Call to Narrow the Gap between Multidrug-Resistant Bacteria in the EU and Development of New Antibacterial Agents. ECDC & EMEA; Solna, Sweden: 2009.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

