In Vitro and In Silico Studies of the Antimicrobial Activity of Prenylated Phenylpropanoids of Green Propolis and Their Derivatives against Oral Bacteria
- PMID: 39200088
- PMCID: PMC11352038
- DOI: 10.3390/antibiotics13080787
In Vitro and In Silico Studies of the Antimicrobial Activity of Prenylated Phenylpropanoids of Green Propolis and Their Derivatives against Oral Bacteria
Abstract
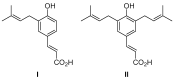
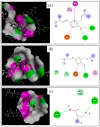
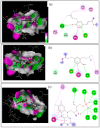
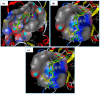
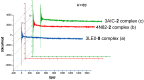
Artepillin C, drupanin, and plicatin B are prenylated phenylpropanoids that naturally occur in Brazilian green propolis. In this study, these compounds and eleven of their derivatives were synthesized and evaluated for their in vitro antimicrobial activity against a representative panel of oral bacteria in terms of their minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values. Plicatin B (2) and its hydrogenated derivative 8 (2',3',7,8-tetrahydro-plicatin B) were the most active compounds. Plicatin B (2) displayed strong activity against all the bacteria tested, with an MIC of 31.2 μg/mL against Streptococcus mutans, S. sanguinis, and S. mitis. On the other hand, compound 8 displayed strong activity against S. mutans, S. salivarius, S. sobrinus, Lactobacillus paracasei (MIC = 62.5 μg/mL), and S. mitis (MIC = 31.2 μg/mL), as well as moderate activity against Enterococcus faecalis and S. sanguinis (MIC = 125 μg/mL). Compounds 2 and 8 displayed bactericidal effects (MBC: MIC ≤ 4) against all the tested bacteria. In silico studies showed that the complexes formed by compounds 2 and 8 with the S. mitis, S. sanguinis, and S. mutans targets (3LE0, 4N82, and 3AIC, respectively) had energy score values similar to those of the native S. mitis, S. sanguinis, and S. mutans ligands due to the formation of strong hydrogen bonds. Moreover, all the estimated physicochemical parameters satisfied the drug-likeness criteria without violating the Lipinski, Veber, and Egan rules, so these compounds are not expected to cause problems with oral bioavailability and pharmacokinetics. Compounds 2 and 8 also had suitable ADMET parameters, as the online server pkCSM calculates. These results make compounds 2 and 8 good candidates as antibacterial agents against oral bacteria.
Keywords: artepillin C; molecular docking; oral bacteria; oral pathogens; plicatin B.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Antimicrobial Activity of the Essential Oil of Plectranthus neochilus against Cariogenic Bacteria.Evid Based Complement Alternat Med. 2015;2015:102317. doi: 10.1155/2015/102317. Epub 2015 Jun 16. Evid Based Complement Alternat Med. 2015. PMID: 26161115 Free PMC article.
-
Antimicrobial Activity of Monoketone Curcuminoids Against Cariogenic Bacteria.Chem Biodivers. 2018 Aug;15(8):e1800216. doi: 10.1002/cbdv.201800216. Epub 2018 Jul 15. Chem Biodivers. 2018. PMID: 29869833
-
Anticariogenic Activity of Three Essential Oils from Brazilian Piperaceae.Pharmaceuticals (Basel). 2022 Aug 6;15(8):972. doi: 10.3390/ph15080972. Pharmaceuticals (Basel). 2022. PMID: 36015120 Free PMC article.
-
Preparation and antimicrobial activity of gelatin microparticles containing propolis against oral pathogens.Drug Dev Ind Pharm. 2006 Feb;32(2):229-38. doi: 10.1080/03639040500466312. Drug Dev Ind Pharm. 2006. PMID: 16537203
-
Role of aqueous extract of morinda citrifolia (Indian noni) ripe fruits in inhibiting dental caries-causing streptococcus mutans and streptococcus mitis.J Dent (Tehran). 2014 Nov;11(6):703-10. Epub 2014 Nov 30. J Dent (Tehran). 2014. PMID: 25628701 Free PMC article. Review.
Cited by
-
Inhibitory effects of carvacrol on glucansucrase from Streptococcus mutans and salivary α-amylase: in silico and in vitro studies.Turk J Biol. 2025 Jan 8;49(1):92-101. doi: 10.55730/1300-0152.2727. eCollection 2025. Turk J Biol. 2025. PMID: 40104578 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

