Should Pregnant Women Consume Probiotics to Combat Endocrine-Disrupting Chemical-Induced Health Risks to Their Unborn Offspring?
- PMID: 39200093
- PMCID: PMC11351870
- DOI: 10.3390/biomedicines12081628
Should Pregnant Women Consume Probiotics to Combat Endocrine-Disrupting Chemical-Induced Health Risks to Their Unborn Offspring?
Abstract
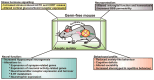
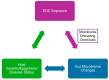
Endocrine-disrupting chemicals (EDCs) have become so pervasive in our environment and daily lives that it is impossible to avoid contact with such compounds, including pregnant women seeking to minimize exposures to themselves and their unborn children. Developmental exposure of humans and rodent models to bisphenol A (BPA) and other EDCs is linked to increased anxiogenic behaviors, learning and memory deficits, and decreased socio-sexual behaviors. Prenatal exposure to BPA and other EDCs leads to longstanding and harmful effects on gut microbiota with reductions in beneficial bacteria, i.e., gut dysbiosis, and such microbial changes are linked to host changes in fecal metabolites, including those involved in carbohydrate metabolism and synthesis, and neurobehavioral alterations in adulthood, in particular, social and cognitive deficits. Gut dysbiosis is increasingly being recognized as a key driver of a myriad of diseases, ranging from metabolic, cardiovascular, reproductive, and neurobehavioral disorders via the gut-microbiome-brain axis. Thus, EDCs might induce indirect effects on physical and mental health by acting as microbiome-disrupting chemicals. Findings raise the important question as to whether pregnant women should consume a probiotic supplement to mitigate pernicious effects of EDCs, especially BPA, on themselves and their unborn offspring. Current studies investigating the effects of maternal probiotic supplementation on pregnant women's health and that of their unborn offspring will be reviewed. Data will inform on the potential application of probiotic supplementation to reverse harmful effects of EDCs, especially BPA, in pregnant women unwittingly exposed to these compounds and striving to give their offspring the best start in life.
Keywords: DOHaD; bisphenol A; genistein; gestation; gut microbiota; in utero; microbiome; neurobehavioral disorders; xenoestrogens.
Conflict of interest statement
Rosenfeld does not have any personal or financial conflicts of interest with respect to the work reported in this article.
Figures


Similar articles
-
Developmental exposure of California mice to endocrine disrupting chemicals and potential effects on the microbiome-gut-brain axis at adulthood.Sci Rep. 2020 Jul 2;10(1):10902. doi: 10.1038/s41598-020-67709-9. Sci Rep. 2020. PMID: 32616744 Free PMC article.
-
Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model.Gut Microbes. 2016 Nov;7(6):471-485. doi: 10.1080/19490976.2016.1234657. Epub 2016 Sep 13. Gut Microbes. 2016. PMID: 27624382 Free PMC article.
-
Gut microbiota contributes to bisphenol A-induced maternal intestinal and placental apoptosis, oxidative stress, and fetal growth restriction in pregnant ewe model by regulating gut-placental axis.Microbiome. 2024 Feb 17;12(1):28. doi: 10.1186/s40168-024-01749-5. Microbiome. 2024. PMID: 38365714 Free PMC article.
-
Gut Dysbiosis in Animals Due to Environmental Chemical Exposures.Front Cell Infect Microbiol. 2017 Sep 8;7:396. doi: 10.3389/fcimb.2017.00396. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28936425 Free PMC article. Review.
-
Effects of Phytoestrogens on the Developing Brain, Gut Microbiota, and Risk for Neurobehavioral Disorders.Front Nutr. 2019 Aug 29;6:142. doi: 10.3389/fnut.2019.00142. eCollection 2019. Front Nutr. 2019. PMID: 31555657 Free PMC article. Review.
References
-
- Braun J.M., Muckle G., Arbuckle T., Bouchard M.F., Fraser W.D., Ouellet E., Séguin J.R., Oulhote Y., Webster G.M., Lanphear B.P. Associations of prenatal urinary bisphenol A concentrations with child behaviors and cognitive abilities. Environ. Health Perspect. 2017;125:067008. doi: 10.1289/EHP984. - DOI - PMC - PubMed
-
- Perera F., Nolte E.L.R., Wang Y., Margolis A.E., Calafat A.M., Wang S., Garcia W., Hoepner L.A., Peterson B.S., Rauh V., et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ. Res. 2016;151:195–202. doi: 10.1016/j.envres.2016.07.028. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

