The Aggregation of α-Synuclein in the Dorsomedial Striatum Significantly Impairs Cognitive Flexibility in Parkinson's Disease Mice
- PMID: 39200099
- PMCID: PMC11351470
- DOI: 10.3390/biomedicines12081634
The Aggregation of α-Synuclein in the Dorsomedial Striatum Significantly Impairs Cognitive Flexibility in Parkinson's Disease Mice
Abstract
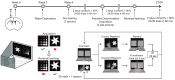
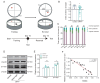
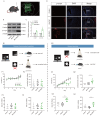
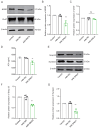
This study focused on α-synuclein (α-syn) aggregation in the dorsomedial striatum (DMS) so as to investigate its role in the cognitive flexibility of Parkinson's disease (PD). Here, we investigated the cognitive flexibility by assessing reversal learning abilities in MPTP-induced subacute PD model mice and in C57BL/6J mice with α-syn aggregation in the DMS induced by adenovirus (AAV-SNCA) injection, followed by an analysis of the target protein's expression and distribution. PD mice exhibited impairments in reversal learning, positively correlated with the expression of phosphorylated α-syn in the DMS. Furthermore, the mice in the AAV-SNCA group exhibited reversal learning deficits and a reduction in acetylcholine levels, accompanied by protein alterations within the DMS. Notably, the administration of a muscarinic receptor 1 (M1R) agonist was able to alleviate the aforementioned phenomenon. These findings suggest that the impaired cognitive flexibility in PD may be attributed to the diminished activation of acetylcholine to M1R caused by α-syn aggregation.
Keywords: Parkinson’s disease; acetylcholine; cognitive flexibility; muscarinic receptor 1; reversal learning; α-synuclein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
α-Synuclein Selectively Impairs Motor Sequence Learning and Value Sensitivity: Reversal by the Adenosine A2A Receptor Antagonists.Cereb Cortex. 2022 Feb 8;32(4):808-823. doi: 10.1093/cercor/bhab244. Cereb Cortex. 2022. PMID: 34339491
-
Dose-related biphasic effect of the Parkinson's disease neurotoxin MPTP, on the spread, accumulation, and toxicity of α-synuclein.Neurotoxicology. 2021 May;84:41-52. doi: 10.1016/j.neuro.2021.02.001. Epub 2021 Feb 4. Neurotoxicology. 2021. PMID: 33549656
-
Synapsin III deficiency hampers α-synuclein aggregation, striatal synaptic damage and nigral cell loss in an AAV-based mouse model of Parkinson's disease.Acta Neuropathol. 2018 Oct;136(4):621-639. doi: 10.1007/s00401-018-1892-1. Epub 2018 Jul 25. Acta Neuropathol. 2018. PMID: 30046897
-
The Impact of SNCA Variations and Its Product Alpha-Synuclein on Non-Motor Features of Parkinson's Disease.Life (Basel). 2021 Aug 9;11(8):804. doi: 10.3390/life11080804. Life (Basel). 2021. PMID: 34440548 Free PMC article. Review.
-
The contribution of alpha synuclein to neuronal survival and function - Implications for Parkinson's disease.J Neurochem. 2016 May;137(3):331-59. doi: 10.1111/jnc.13570. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26852372 Free PMC article. Review.
References
-
- Santos-Garcia D., de Deus Fonticoba T., Cores Bartolome C., Feal Painceiras M.J., Iniguez-Alvarado M.C., Garcia Diaz I., Jesus S., Buongiorno M.T., Planellas L., Cosgaya M., et al. Staging Parkinson’s Disease According to the MNCD (Motor/Non-motor/Cognition/Dependency) Classification Correlates with Disease Severity and Quality of Life. J. Park. Dis. 2023;13:379–402. doi: 10.3233/JPD-225073. - DOI - PMC - PubMed
-
- Wang Z., Flores I., Donahue E.K., Lundquist A.J., Guo Y., Petzinger G.M., Jakowec M.W., Holschneider D.P. Cognitive flexibility deficits in rats with dorsomedial striatal 6-hydroxydopamine lesions tested using a three-choice serial reaction time task with reversal learning. Neuroreport. 2020;31:1055–1064. doi: 10.1097/WNR.0000000000001509. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

