Evaluation of Anti-CAR Linker mAbs for CAR T Monitoring after BiTEs/bsAbs and CAR T-Cell Pretreatment
- PMID: 39200107
- PMCID: PMC11351819
- DOI: 10.3390/biomedicines12081641
Evaluation of Anti-CAR Linker mAbs for CAR T Monitoring after BiTEs/bsAbs and CAR T-Cell Pretreatment
Abstract
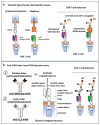
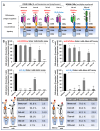
For the monitoring of chimeric antigen receptor (CAR) T-cell therapies, antigen-based CAR detection methods are usually applied. However, for each target-antigen, a separate detection system is required. Furthermore, when monitored CAR T-cells in the blood of patients treated with bispecific antibodies or T-cell engagers (bsAbs/BiTEs) recognize the same antigen, these methods produce false-positive results in clinical diagnostics. Anti-CAR-linker monoclonal antibodies (mAbs) targeting the linker sequence between the variable domains of the antigen binding CAR fragment promise a universal and unbiased CAR detection. To test this, we analyzed clinical specimens of all BCMA- and CD19-targeting CAR T-cell products currently approved for clinical use. We found a highly specific and sensitive CAR detection using anti-CAR-linker mAb in blood cells from patients treated with Ide-cel, Tisa-cel, Axi-cel, Brexu-cel, and Liso-cel. For Ide-cel and Tisa-cel, the sensitivity was significantly lower compared to that for antigen-based CAR detection assays. Strikingly, the specificity of anti-CAR linker mAb was not affected by the simultaneous presence of bispecific blinatumomab or teclistamab for Axi-cel, Brexu-cel, Liso-cel, or Ide-cel, respectively. Cilta-cel (containing a monomeric G4S-CAR linker) could not be detected by anti-CAR linker mAb. In conclusion, anti-CAR-linker mAbs are highly specific and useful for CAR T-cell monitoring but are not universally applicable.
Keywords: BiTE; CAR; CAR T-cell monitoring; anti-CAR-linker mAbs; bispecific antibody; combination treatments; detection; diagnostic.
Conflict of interest statement
Authors M.M., V.V. and U.P. were employed by the companies Janssen and BMS. Author U.K. was employed as consultant by the companies AstraZeneca, Affimed, Glycostem, GammaDelta, and Zelluna. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cost-Effectiveness of Chimeric Antigen Receptor T Cell Therapy in Patients with Relapsed or Refractory Large B Cell Lymphoma: No Impact of Site of Care.Adv Ther. 2022 Aug;39(8):3560-3577. doi: 10.1007/s12325-022-02188-0. Epub 2022 Jun 11. Adv Ther. 2022. PMID: 35689726 Free PMC article.
-
Real-World Outcomes with Chimeric Antigen Receptor T Cell Therapies in Large B Cell Lymphoma: A Systematic Review and Meta-Analysis.Transplant Cell Ther. 2024 Jan;30(1):77.e1-77.e15. doi: 10.1016/j.jtct.2023.10.017. Epub 2023 Oct 27. Transplant Cell Ther. 2024. PMID: 37890589
-
Droplet digital PCR allows vector copy number assessment and monitoring of experimental CAR T cells in murine xenograft models or approved CD19 CAR T cell-treated patients.J Transl Med. 2021 Jun 21;19(1):265. doi: 10.1186/s12967-021-02925-z. J Transl Med. 2021. PMID: 34154602 Free PMC article.
-
Accurate In-Vivo Quantification of CD19 CAR-T Cells after Treatment with Axicabtagene Ciloleucel (Axi-Cel) and Tisagenlecleucel (Tisa-Cel) Using Digital PCR.Cancers (Basel). 2020 Jul 20;12(7):1970. doi: 10.3390/cancers12071970. Cancers (Basel). 2020. PMID: 32698364 Free PMC article.
-
Ciltacabtagene autoleucel: The second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma.Front Immunol. 2022 Sep 2;13:991092. doi: 10.3389/fimmu.2022.991092. eCollection 2022. Front Immunol. 2022. PMID: 36119032 Free PMC article. Review.
Cited by
-
Bispecific Antibodies as Bridging to BCMA CAR-T Cell Therapy for Relapsed/Refractory Multiple Myeloma.Blood Cancer Discov. 2025 Jan 8;6(1):38-54. doi: 10.1158/2643-3230.BCD-24-0118. Blood Cancer Discov. 2025. PMID: 39441177 Free PMC article.
References
-
- Westin J.R., Kersten M.J., Salles G., Abramson J.S., Schuster S.J., Locke F.L., Andreadis C. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am. J. Hematol. 2021;96:1295–1312. doi: 10.1002/ajh.26301. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

