Investigation of the Protective Effects of Magnesium on Bupivacaine-Induced Toxicity at the Level of Colon Cell Culture
- PMID: 39200118
- PMCID: PMC11351263
- DOI: 10.3390/biomedicines12081652
Investigation of the Protective Effects of Magnesium on Bupivacaine-Induced Toxicity at the Level of Colon Cell Culture
Abstract
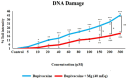
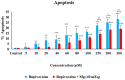
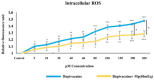
The primary objective of this in vitro study was to prevent the risk of toxicity associated with bupivacaine, widely used in clinical practice, by using magnesium (Mg), a readily available and cost-effective element, as an adjuvant. We hypothesized that Mg might exhibit a protective effect against cytotoxicity in a colon cell culture model under conditions of bupivacaine-induced LAST. Our secondary aim was to investigate its effect on genotoxicity, apoptosis, and iROS. CCD-18Co cells were used in our study. Control group (group C), Bupivacaine group (group B), Magnesium group (group M), and Bupivacaine+Mg group (group BM) were created. The viability of CCD-18Co cells incubated for 24 h in group C was determined to be 100%. These cells were evenly divided, and bupivacaine was administered to group B at concentrations of 5 to 300 μM. In group M, doses of Mg at 0.625 to 320 mEq were added. It was determined that the maximum viability was observed at a Mg dose of 40 mEq (p < 0.05). In group BM, bupivacaine was administered at the same concentrations in combination with Mg (40 mEq), and cell viability was measured. DNA damage, apoptosis, and iROS were assessed at concentrations of bupivacaine by administering 40 mEq Mg. In group B, viability decreased dose-dependently in CCD-18Co (p < 0.05, p < 0.01, p < 0.001). In group BM, the viability decreased in cells at increasing concentrations compared to group C (p < 0.05, p < 0.01, p < 0.001), but the viability was affected positively compared to group B (p < 0.05). In group B, DNA damage increased (p < 0.05, p < 0.001). In group BM, DNA damage increased (p < 0.05, p < 0.001). However, in group BM, DNA damage levels were reduced compared to group B (p < 0.05, p < 0.01). In group B, apoptosis increased (p < 0.05, p < 0.001); in group BM, apoptosis increased (p < 0.001) compared to group C. However, in group BM, apoptosis decreased compared to group B (p< 0.05). iROS increased in group B (p < 0.05, p < 0.01, p < 0.01) and group BM (p < 0.05, p < 0.01, p < 0.001) compared to the group C. However, in group BM, iROS decreased in comparison to group B (p < 0.05). In conclusion, Mg exhibits a protective effect against bupivacaine-induced toxicity.
Keywords: DNA damage; apoptosis; bupivacaine; cytotoxicity; magnesium.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Figures


Similar articles
-
Investigation of the Effect of Amniomax on Lidocaine-Induced Toxicity in Healthy Colon Cell Culture.Biomedicines. 2025 Apr 29;13(5):1074. doi: 10.3390/biomedicines13051074. Biomedicines. 2025. PMID: 40426902 Free PMC article.
-
Efficacy of Magnesium Sulfate as an Adjuvant to Bupivacaine in Transversus Abdominis Plane Block for Abdominal Hysterectomy Surgeries.Cureus. 2023 Apr 5;15(4):e37156. doi: 10.7759/cureus.37156. eCollection 2023 Apr. Cureus. 2023. PMID: 37159770 Free PMC article.
-
Bupivacaine and Lidocaine Induce Apoptosis in Osteosarcoma Tumor Cells.Clin Orthop Relat Res. 2021 Jan 1;479(1):180-194. doi: 10.1097/CORR.0000000000001510. Clin Orthop Relat Res. 2021. PMID: 33009230 Free PMC article.
-
Comparative study of caudal clonidine and midazolam added to bupivacaine during infra-umbilical surgeries in children.J Anaesthesiol Clin Pharmacol. 2017 Apr-Jun;33(2):241-247. doi: 10.4103/0970-9185.209739. J Anaesthesiol Clin Pharmacol. 2017. PMID: 28781453 Free PMC article.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
References
-
- Neal J.M., Barrington M.J., Fettiplace M.R., Gitman M., Memtsoudis S.G., Mörwald E.E., Rubin D.S., Weinberg G. The Third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on Local Anesthetic Systemic Toxicity. Reg. Anesth. Pain Med. 2018;43:113–123. doi: 10.1097/aap.0000000000000720. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

