Leveraging Hypotension Prediction Index to Forecast LPS-Induced Acute Lung Injury and Inflammation in a Porcine Model: Exploring the Role of Hypoxia-Inducible Factor in Circulatory Shock
- PMID: 39200130
- PMCID: PMC11351327
- DOI: 10.3390/biomedicines12081665
Leveraging Hypotension Prediction Index to Forecast LPS-Induced Acute Lung Injury and Inflammation in a Porcine Model: Exploring the Role of Hypoxia-Inducible Factor in Circulatory Shock
Abstract
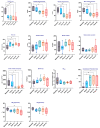
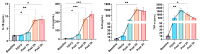
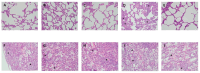
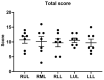
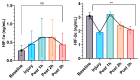
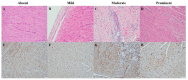
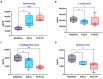
Acute respiratory distress syndrome (ARDS) is a critical illness in critically unwell patients, characterized by refractory hypoxemia and shock. This study evaluates an early detection tool and investigates the relationship between hypoxia and circulatory shock in ARDS, to improve diagnostic precision and therapy customization. We used a porcine model, inducing ARDS with mechanical ventilation and intratracheal plus intravenous lipopolysaccharide (LPS) injection. Hemodynamic changes were monitored using an Acumen IQ sensor and a ForeSight Elite sensor connected to the HemoSphere platform. We evaluated tissue damage, inflammatory response, and hypoxia-inducible factor (HIF) alterations using enzyme-linked immunosorbent assay and immunohistochemistry. The results showed severe hypotension and increased heart rates post-LPS exposure, with a notable rise in the hypotension prediction index (HPI) during acute lung injury (p = 0.024). Tissue oxygen saturation dropped considerably in the right brain region. Interestingly, post-injury HIF-2α levels were lower at the end of the experiment. Our findings imply that the HPI can effectively predict ARDS-related hypotension. HIF expression levels may serve as possible markers of rapid ARDS progression. Further research should be conducted on the clinical value of this novel approach in critical care, as well as the relationship between the HIF pathway and ARDS-associated hypotension.
Keywords: hemodynamic monitoring; hypotension; hypotension prediction index; hypoxia-inducible factor; lipopolysaccharide; lung injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

