A Highly Standardized Pre-Clinical Porcine Wound Healing Model Powered by Semi-Automated Histological Analysis
- PMID: 39200162
- PMCID: PMC11351733
- DOI: 10.3390/biomedicines12081697
A Highly Standardized Pre-Clinical Porcine Wound Healing Model Powered by Semi-Automated Histological Analysis
Abstract
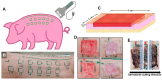
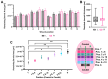
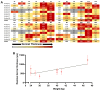
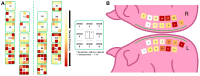
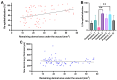
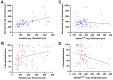
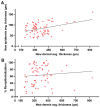
The wound-healing process is a physiological response that begins after a disruption to the integrity of tissues present in the skin. To understand the intricacies involved in this process, many groups have tried to develop different in vitro models; however, the lack of a systemic response has, until this day, been the major barrier to the establishment of these models as the main study platform. Therefore, in vivo models are still the most common system for studying healing responses following different treatments, especially porcine models, which share several morphological similarities to the human skin. In this work, we developed a porcine excisional wound model and used semi-automated software as a strategy to generate quantitative morphometric results of healing responses by specific tissues and compartments. Our aim was to extract the most information from the model while producing reliable, reproducible, and standardized results. In order to achieve this, we established a 7-day treatment using a bacterial cellulose dressing as our standard for all the analyzed wounds. The thickness of the residual dermis under the wound (DUtW) bed was shown to influence the healing outcome, especially for the regeneration of epidermal tissue, including the wound closure rate. The analysis of the DUtW throughout the entire dorsal region of the animals opened up the possibility of establishing a map that will facilitate the experimental design of future works, increasing their standardization and reproducibility and ultimately reducing the number of animals needed. Thus, the developed model, together with the automated morphometric analysis approach used, offers the possibility to generate robust quantitative results with a rapid turnaround time while allowing the study of multiple extra morphometric parameters, creating a more holistic analysis.
Keywords: burns; reproducibility; semi-automated analysis; standardization; translational model; wound healing model.
Conflict of interest statement
This study was funded by Evomedis GmbH, Austria, of which the author, Martin Funk, is a part. Fabian Kukla and Thomas Lemarchand were employed by the TPL Path Labs GmbH. This had no influence on the collection, analysis, interpretation, or submission of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial of financial relationship that could be constructed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

