Deep Learning-Based Real-Time Organ Localization and Transit Time Estimation in Wireless Capsule Endoscopy
- PMID: 39200169
- PMCID: PMC11351118
- DOI: 10.3390/biomedicines12081704
Deep Learning-Based Real-Time Organ Localization and Transit Time Estimation in Wireless Capsule Endoscopy
Abstract
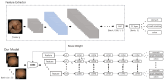
Background: Wireless capsule endoscopy (WCE) has significantly advanced the diagnosis of gastrointestinal (GI) diseases by allowing for the non-invasive visualization of the entire small intestine. However, machine learning-based methods for organ classification in WCE often rely on color information, leading to decreased performance when obstacles such as food debris are present. This study proposes a novel model that integrates convolutional neural networks (CNNs) and long short-term memory (LSTM) networks to analyze multiple frames and incorporate temporal information, ensuring that it performs well even when visual information is limited.
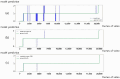
Methods: We collected data from 126 patients using PillCam™ SB3 (Medtronic, Minneapolis, MN, USA), which comprised 2,395,932 images. Our deep learning model was trained to identify organs (stomach, small intestine, and colon) using data from 44 training and 10 validation cases. We applied calibration using a Gaussian filter to enhance the accuracy of detecting organ boundaries. Additionally, we estimated the transit time of the capsule in the gastric and small intestine regions using a combination of a convolutional neural network (CNN) and a long short-term memory (LSTM) designed to be aware of the sequence information of continuous videos. Finally, we evaluated the model's performance using WCE videos from 72 patients.
Results: Our model demonstrated high performance in organ classification, achieving an accuracy, sensitivity, and specificity of over 95% for each organ (stomach, small intestine, and colon), with an overall accuracy and F1-score of 97.1%. The Matthews Correlation Coefficient (MCC) and Geometric Mean (G-mean) were used to evaluate the model's performance on imbalanced datasets, achieving MCC values of 0.93 for the stomach, 0.91 for the small intestine, and 0.94 for the colon, and G-mean values of 0.96 for the stomach, 0.95 for the small intestine, and 0.97 for the colon. Regarding the estimation of gastric and small intestine transit times, the mean time differences between the model predictions and ground truth were 4.3 ± 9.7 min for the stomach and 24.7 ± 33.8 min for the small intestine. Notably, the model's predictions for gastric transit times were within 15 min of the ground truth for 95.8% of the test dataset (69 out of 72 cases). The proposed model shows overall superior performance compared to a model using only CNN.
Conclusions: The combination of CNN and LSTM proves to be both accurate and clinically effective for organ classification and transit time estimation in WCE. Our model's ability to integrate temporal information allows it to maintain high performance even in challenging conditions where color information alone is insufficient. Including MCC and G-mean metrics further validates the robustness of our approach in handling imbalanced datasets. These findings suggest that the proposed method can significantly improve the diagnostic accuracy and efficiency of WCE, making it a valuable tool in clinical practice for diagnosing and managing GI diseases.
Keywords: deep learning; gastrointestinal transit; wireless capsule endoscopy.
Conflict of interest statement
Gwiseong Moon, Jung-Hwan Park, Yoon Kim and Hyun-Soo Choi were employed by the company Ziovision Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
Wireless capsule endoscopy multiclass classification using three-dimensional deep convolutional neural network model.Biomed Eng Online. 2023 Dec 15;22(1):124. doi: 10.1186/s12938-023-01186-9. Biomed Eng Online. 2023. PMID: 38098015 Free PMC article.
-
Revealing the Boundaries of Selected Gastro-Intestinal (GI) Organs by Implementing CNNs in Endoscopic Capsule Images.Diagnostics (Basel). 2023 Feb 23;13(5):865. doi: 10.3390/diagnostics13050865. Diagnostics (Basel). 2023. PMID: 36900009 Free PMC article.
-
Small Bowel Detection for Wireless Capsule Endoscopy Using Convolutional Neural Networks with Temporal Filtering.Diagnostics (Basel). 2022 Jul 31;12(8):1858. doi: 10.3390/diagnostics12081858. Diagnostics (Basel). 2022. PMID: 36010210 Free PMC article.
-
High pooled performance of convolutional neural networks in computer-aided diagnosis of GI ulcers and/or hemorrhage on wireless capsule endoscopy images: a systematic review and meta-analysis.Gastrointest Endosc. 2021 Feb;93(2):356-364.e4. doi: 10.1016/j.gie.2020.07.038. Epub 2020 Jul 25. Gastrointest Endosc. 2021. PMID: 32721487
-
Computer vision-based solutions to overcome the limitations of wireless capsule endoscopy.J Med Eng Technol. 2023 Apr-May;47(4):242-261. doi: 10.1080/03091902.2024.2302025. Epub 2024 Jan 22. J Med Eng Technol. 2023. PMID: 38231042 Review.
Cited by
-
Edge Artificial Intelligence Device in Real-Time Endoscopy for Classification of Gastric Neoplasms: Development and Validation Study.Biomimetics (Basel). 2024 Dec 22;9(12):783. doi: 10.3390/biomimetics9120783. Biomimetics (Basel). 2024. PMID: 39727787 Free PMC article.
-
Deep Learning Models for Anatomical Location Classification in Esophagogastroduodenoscopy Images and Videos: A Quantitative Evaluation with Clinical Data.Diagnostics (Basel). 2024 Oct 23;14(21):2360. doi: 10.3390/diagnostics14212360. Diagnostics (Basel). 2024. PMID: 39518328 Free PMC article.
References
-
- Ding Z., Shi H., Zhang H., Meng L., Fan M., Han C., Zhang K., Ming F., Xie X., Liu H., et al. Gastroenterologist-Level Identification of Small-Bowel Diseases and Normal Variants by Capsule Endoscopy Using a Deep-Learning Model. Gastroenterology. 2019;157:1044–1054.e5. doi: 10.1053/j.gastro.2019.06.025. - DOI - PubMed
LinkOut - more resources
Full Text Sources

