Differential Mitochondrial Bioenergetics in Neurons and Astrocytes Following Ischemia-Reperfusion Injury and Hypothermia
- PMID: 39200170
- PMCID: PMC11352110
- DOI: 10.3390/biomedicines12081705
Differential Mitochondrial Bioenergetics in Neurons and Astrocytes Following Ischemia-Reperfusion Injury and Hypothermia
Abstract
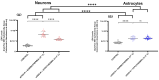
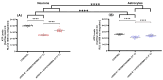
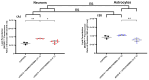
The close interaction between neurons and astrocytes has been extensively studied. However, the specific behavior of these cells after ischemia-reperfusion injury and hypothermia remains poorly characterized. A growing body of evidence suggests that mitochondria function and putative transference between neurons and astrocytes may play a fundamental role in adaptive and homeostatic responses after systemic insults such as cardiac arrest, which highlights the importance of a better understanding of how neurons and astrocytes behave individually in these settings. Brain injury is one of the most important challenges in post-cardiac arrest syndrome, and therapeutic hypothermia remains the single, gold standard treatment for neuroprotection after cardiac arrest. In our study, we modeled ischemia-reperfusion injury by using in vitro enhanced oxygen-glucose deprivation and reperfusion (eOGD-R) and subsequent hypothermia (HPT) (31.5 °C) to cell lines of neurons (HT-22) and astrocytes (C8-D1A) with/without hypothermia. Using cell lysis (LDH; lactate dehydrogenase) as a measure of membrane integrity and cell viability, we found that neurons were more susceptible to eOGD-R when compared with astrocytes. However, they benefited significantly from HPT, while the HPT effect after eOGD-R on astrocytes was negligible. Similarly, eOGD-R caused a more significant reduction in adenosine triphosphate (ATP) in neurons than astrocytes, and the ATP-enhancing effects from HPT were more prominent in neurons than astrocytes. In both neurons and astrocytes, measurement of reactive oxygen species (ROS) revealed higher ROS output following eOGD-R, with a non-significant trend of differential reduction observed in neurons. HPT after eOGD-R effectively downregulated ROS in both cells; however, the effect was significantly more effective in neurons. Lipid peroxidation was higher after eOGD-R in neurons, while in astrocytes, the increase was not statistically significant. Interestingly, HPT had similar effects on the reduction in lipoperoxidation after eOGD-R with both types of cells. While glutathione (GSH) levels were downregulated after eOGD-R in both cells, HPT enhanced GSH in astrocytes, but worsened GSH in neurons. In conclusion, neuron and astrocyte cultures respond differently to eOGD-R and eOGD-R + HTP treatments. Neurons showed higher sensitivity to ischemia-reperfusion insults than astrocytes; however, they benefited more from HPT therapy. These data suggest that given the differential effects from HPT in neurons and astrocytes, future therapeutic developments could potentially enhance HPT outcomes by means of neuronal and astrocytic targeted therapies.
Keywords: astrocytes; cardiac arrest; hypothermia; ischemia-reperfusion injury; neurons; neuroprotection.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

