The Neuroprotective Effect of Neural Cell Adhesion Molecule L1 in the Hippocampus of Aged Alzheimer's Disease Model Mice
- PMID: 39200191
- PMCID: PMC11351965
- DOI: 10.3390/biomedicines12081726
The Neuroprotective Effect of Neural Cell Adhesion Molecule L1 in the Hippocampus of Aged Alzheimer's Disease Model Mice
Erratum in
-
Correction: Aksic et al. The Neuroprotective Effect of Neural Cell Adhesion Molecule L1 in the Hippocampus of Aged Alzheimer's Disease Model Mice. Biomedicines 2024, 12, 1726.Biomedicines. 2025 Jan 24;13(2):290. doi: 10.3390/biomedicines13020290. Biomedicines. 2025. PMID: 39899285 Free PMC article.
Abstract
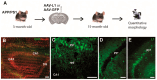
Alzheimer's disease (AD) is a severe neurodegenerative disorder and the most common form of dementia, causing the loss of cognitive function. Our previous study has shown, using a doubly mutated mouse model of AD (APP/PS1), that the neural adhesion molecule L1 directly binds amyloid peptides and decreases plaque load and gliosis when injected as an adeno-associated virus construct (AAV-L1) into APP/PS1 mice. In this study, we microinjected AAV-L1, using a Hamilton syringe, directly into the 3-month-old APP/PS1 mouse hippocampus and waited for a year until significant neurodegeneration developed. We stereologically counted the principal neurons and parvalbumin-positive interneurons in the hippocampus, estimated the density of inhibitory synapses around principal cells, and compared the AAV-L1 injection models with control injections of green fluorescent protein (AAV-GFP) and the wild-type hippocampus. Our results show that there is a significant loss of granule cells in the dentate gyrus of the APP/PS1 mice, which was improved by AAV-L1 injection, compared with the AAV-GFP controls (p < 0.05). There is also a generalized loss of parvalbumin-positive interneurons in the hippocampus of APP/PS1 mice, which is ameliorated by AAV-L1 injection, compared with the AAV-GFP controls (p < 0.05). Additionally, AAV-L1 injection promotes the survival of inhibitory synapses around the principal cells compared with AAV-GFP controls in all three hippocampal subfields (p < 0.01). Our results indicate that L1 promotes neuronal survival and protects the synapses in an AD mouse model, which could have therapeutic implications.
Keywords: APP/PS1 mice; Alzheimer’s disease; GABAergic interneurons; adhesion molecule L1; hippocampus; synapses.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Adhesion molecule L1 binds to amyloid beta and reduces Alzheimer's disease pathology in mice.Neurobiol Dis. 2013 Aug;56:104-15. doi: 10.1016/j.nbd.2013.04.014. Epub 2013 Apr 29. Neurobiol Dis. 2013. PMID: 23639788
-
Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease.Neuropharmacology. 2014 Jan;76 Pt A:57-67. doi: 10.1016/j.neuropharm.2013.08.005. Epub 2013 Aug 21. Neuropharmacology. 2014. PMID: 23973293
-
Effect of soluble amyloid precursor protein-alpha on adult hippocampal neurogenesis in a mouse model of Alzheimer's disease.Mol Brain. 2022 Jan 3;15(1):5. doi: 10.1186/s13041-021-00889-1. Mol Brain. 2022. PMID: 34980189 Free PMC article.
-
6-Methyluracil derivatives as acetylcholinesterase inhibitors for treatment of Alzheimer's disease.Int J Risk Saf Med. 2015;27 Suppl 1:S69-71. doi: 10.3233/JRS-150694. Int J Risk Saf Med. 2015. PMID: 26639718
-
Protective effect of potassium 2-(l-hydroxypentyl)-benzoate on hippocampal neurons, synapses and dystrophic axons in APP/PS1 mice.Psychopharmacology (Berl). 2019 Sep;236(9):2761-2771. doi: 10.1007/s00213-019-05251-x. Epub 2019 Jun 4. Psychopharmacology (Berl). 2019. PMID: 31165206
Cited by
-
Correction: Aksic et al. The Neuroprotective Effect of Neural Cell Adhesion Molecule L1 in the Hippocampus of Aged Alzheimer's Disease Model Mice. Biomedicines 2024, 12, 1726.Biomedicines. 2025 Jan 24;13(2):290. doi: 10.3390/biomedicines13020290. Biomedicines. 2025. PMID: 39899285 Free PMC article.
-
Increased expression of the neuroplastin 65 protein is involved in neurofibrillary tangles and amyloid beta plaques in Alzheimer's disease.World J Psychiatry. 2025 Jun 19;15(6):105751. doi: 10.5498/wjp.v15.i6.105751. eCollection 2025 Jun 19. World J Psychiatry. 2025. PMID: 40574756 Free PMC article.
References
LinkOut - more resources
Full Text Sources

