The Humoral Immune Response against Human Endogenous Retroviruses in Celiac Disease: A Case-Control Study
- PMID: 39200275
- PMCID: PMC11351412
- DOI: 10.3390/biomedicines12081811
The Humoral Immune Response against Human Endogenous Retroviruses in Celiac Disease: A Case-Control Study
Abstract
Background: Celiac disease (CD) is an immune-mediated disease characterized by disruptions of the small intestine. Factors such as viral and bacterial infections can trigger CD. Recently, the reactivation of Human Endogenous Retroviruses (HERVs) has also been implicated, but little is known about their specific role in patients with celiac disease.
Methods: The purpose of this study is to explore the humoral immune response mounted against epitopes derived from the envelope portion of three families of HERVs (HERV-K, HERV-H, and HERV-W) in CD patients. Reactivity against the HERV-K, HERV-H, and HERV-W env-su peptides was tested by indirect ELISAs in plasma of 40 patients with celiac disease and 41 age-matched healthy subjects (HCs).
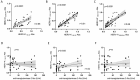
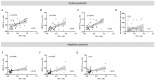
Results: HERV-K, HERV-H, and HERV-W env-su peptides triggered different antibody responses in CD patients compared to HCs, with a stronger reactivity (p = 0.0001).
Conclusions: Present results show, for the first time, that epitopes of HERV-K, HERV-H, and HERV-W are more recognized in patients with CD. Taking into consideration their proinflammatory and autoimmune features, this might suggest that HERVs may contribute to the development of CD or its exacerbation in genetically predisposed subjects. Finally, to elucidate the interplay between gut inflammation and HERVs during the inflammatory process, further studies are required. Those investigations should focus on the expression levels of HERVs and their relationship with the immune response, specifically examining anti-transglutaminase 2 (TG2) antibody levels under both gluten-free and gluten-containing dietary conditions.
Keywords: HERV-H; HERV-K; HERV-W; antigenic peptides; celiac disease; humoral immune response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Antibody Response to HERV-K and HERV-W Envelope Epitopes in Patients with Myasthenia Gravis.Int J Mol Sci. 2023 Dec 28;25(1):446. doi: 10.3390/ijms25010446. Int J Mol Sci. 2023. PMID: 38203616 Free PMC article.
-
A Characterization of the Humoral Immune Response to Human Endogenous Retroviruses and Mycobacterium paratuberculosis in Crohn's Disease.Pathogens. 2025 Apr 7;14(4):361. doi: 10.3390/pathogens14040361. Pathogens. 2025. PMID: 40333136 Free PMC article.
-
Overexpression of endogenous retroviruses in children with celiac disease.Eur J Pediatr. 2021 Aug;180(8):2429-2434. doi: 10.1007/s00431-021-04050-x. Epub 2021 Mar 26. Eur J Pediatr. 2021. PMID: 33772337
-
Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses.Front Immunol. 2018 Sep 10;9:2039. doi: 10.3389/fimmu.2018.02039. eCollection 2018. Front Immunol. 2018. PMID: 30250470 Free PMC article. Review.
-
HERVs in neuropathogenesis.J Neuroimmune Pharmacol. 2010 Sep;5(3):326-35. doi: 10.1007/s11481-010-9214-y. Epub 2010 Apr 27. J Neuroimmune Pharmacol. 2010. PMID: 20422298 Review.
Cited by
-
HERVs Endophenotype in Autism Spectrum Disorder: Human Endogenous Retroviruses, Specific Immunoreactivity, and Disease Association in Different Family Members.Microorganisms. 2024 Dec 24;13(1):9. doi: 10.3390/microorganisms13010009. Microorganisms. 2024. PMID: 39858776 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

