Cardiomyocyte Regeneration in Human Myocarditis
- PMID: 39200277
- PMCID: PMC11352115
- DOI: 10.3390/biomedicines12081814
Cardiomyocyte Regeneration in Human Myocarditis
Abstract
Background: Newly generated cardiomyocytes (NGCs) concur with the recovery of human myocarditis occurring spontaneously in around 50% of cases. However, NGCs decline with age, and their modality of myocardial homing and integration are still unclear.
Methods: We retrospectively assessed NGCs in 213 consecutive patients with endomyocardial biopsy denoting acute myocarditis, with normal coronaries and valves. Tissue samples were processed for histology (H&E), immunohistochemistry for the evaluation of inflammatory infiltrates, immunostaining for alpha-sarcomeric-actin, junctional connexin-43, Ki-67, and phosphorylated STAT3 (p-STAT3), and Western blot (WB) for HMGB1. Frozen samples were analyzed using polymerase chain reaction (PCR) for cardiotropic viruses. Controls included 20 normal surgical biopsies.
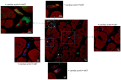
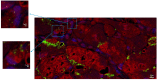
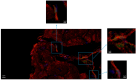
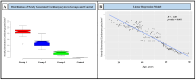
Results: NGCs were defined as small myocytes (diameter < 10 µm) with nuclear positivity to Ki-67 and p-STAT3 and positive immunostaining for cytoplasmic α-sarcomeric actin and connexin-43. Their number/mm2 in relation to age and pathway of integration was evaluated. NGCs crossed the membrane and grew integrated within the empty necrotic myocytes. NGC mean diameter was 6.6 ± 3.34 vs. 22.5 ± 3.11 µm adult cells; their number, in comparison to LVEF, was 86.3 ± 10.3/mm2 in patients between 18 and 40 years, 50.4 ± 13.8/mm2 in those between 41 and 60, and 15.1 ± 5.7/mm2 in those between 61 and 80. Control NGCs' mean diameter was 0.2 ± 0.2 mm2. PCR was positive for viral genomes in 16% of cases; NGCs were not statistically different in viral and non-viral myocarditis. WB analysis revealed a higher expression of HMGB1 in myocarditis compared to myocardial controls.
Conclusions: NGCs are constantly recognizable in acute human myocarditis. Their number declines with age. Their integration within necrotic myocytes allows for the preservation of the cardiac structure and function.
Keywords: STAT3; myocarditis; newly generated cardiomyocytes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Miyawaki A., Obana M., Mitsuhara Y., Orimoto A., Nakayasu Y., Yamashita T., Fukada S., Maeda M., Nakayama H., Fujio Y. Adult Murine Cardiomyocytes Exhibit Regenerative Activity with Cell Cycle Reentry through STAT3 in the Healing Process of Myocarditis. Sci. Rep. 2017;7:1407. doi: 10.1038/s41598-017-01426-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

