Surface Pre-Reacted Glass-Ionomer Eluate Suppresses Osteoclastogenesis through Downregulation of the MAPK Signaling Pathway
- PMID: 39200299
- PMCID: PMC11352117
- DOI: 10.3390/biomedicines12081835
Surface Pre-Reacted Glass-Ionomer Eluate Suppresses Osteoclastogenesis through Downregulation of the MAPK Signaling Pathway
Abstract
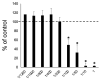
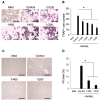
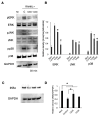
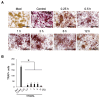
Surface pre-reacted glass-ionomer (S-PRG) is a new bioactive filler utilized for the restoration of decayed teeth by its ability to release six bioactive ions that prevent the adhesion of dental plaque to the tooth surface. Since ionic liquids are reported to facilitate transepithelial penetration, we reasoned that S-PRG applied to root caries could impact the osteoclasts (OCs) in the proximal alveolar bone. Therefore, this study aimed to investigate the effect of S-PRG eluate solution on RANKL-induced OC-genesis and mineral dissolution in vitro. Using RAW264.7 cells as OC precursor cells (OPCs), TRAP staining and pit formation assays were conducted to monitor OC-genesis and mineral dissolution, respectively, while OC-genesis-associated gene expression was measured using quantitative real-time PCR (qPCR). Expression of NFATc1, a master regulator of OC differentiation, and the phosphorylation of MAPK signaling molecules were measured using Western blotting. S-PRG eluate dilutions at 1/200 and 1/400 showed no cytotoxicity to RAW264.7 cells but did significantly suppress both OC-genesis and mineral dissolution. The same concentrations of S-PRG eluate downregulated the RANKL-mediated induction of OCSTAMP and CATK mRNAs, as well as the expression of NFATc1 protein and the phosphorylation of ERK, JNK, and p38. These results demonstrate that S-PRG eluate can downregulate RANKL-induced OC-genesis and mineral dissolution, suggesting that its application to root caries might prevent alveolar bone resorption.
Keywords: S-PRG; TRAP staining; bioactive filler; hydroxyapatite; osteoclast.
Conflict of interest statement
All authors, except T.N., declare no conflicts of interest. T.N. is a member of Shifu inc. which provided the S-PRG materials. Shofu Inc. has been sharing S-PRG filler and S-PGR eluate to all researchers worldwide free of charge. T.N.’s role in this study was limited to providing high-quality S-PGR eluate and developing the research hypothesis together with corresponding authors in strict compliance with conflict of interest guidelines.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

