Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles
- PMID: 39200314
- PMCID: PMC11351396
- DOI: 10.3390/biomedicines12081850
Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles
Abstract
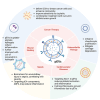
Platelet-derived extracellular vesicles (pEVs) are emerging as pivotal players in numerous physiological and pathological processes, extending beyond their traditional roles in hemostasis and thrombosis. As one of the most abundant vesicle types in human blood, pEVs transport a diverse array of bioactive molecules, including growth factors, cytokines, and clotting factors, facilitating crucial intercellular communication, immune regulation, and tissue healing. The unique ability of pEVs to traverse tissue barriers and their biocompatibility position them as promising candidates for targeted drug delivery and regenerative medicine applications. Recent studies have underscored their involvement in cancer progression, viral infections, wound healing, osteoarthritis, sepsis, cardiovascular diseases, rheumatoid arthritis, and atherothrombosis. For instance, pEVs promote tumor progression and metastasis, enhance tissue repair, and contribute to thrombo-inflammation in diseases such as COVID-19. Despite their potential, challenges remain, including the need for standardized isolation techniques and a comprehensive understanding of their mechanisms of action. Current research efforts are focused on leveraging pEVs for innovative anti-cancer treatments, advanced drug delivery systems, regenerative therapies, and as biomarkers for disease diagnosis and monitoring. This review highlights the necessity of overcoming technical hurdles, refining isolation methods, and establishing standardized protocols to fully unlock the therapeutic potential of pEVs. By understanding the diverse functions and applications of pEVs, we can advance their use in clinical settings, ultimately revolutionizing treatment strategies across various medical fields and improving patient outcomes.
Keywords: angiogenesis; hemostasis; immune modulation; platelet-derived extracellular vesicles (pEVs); regenerative medicine.
Conflict of interest statement
The authors declare that they have no competing interests. The funder had no role in the manuscript submission and publication.
Figures


References
-
- Guerreiro E.M., Kruglik S.G., Swamy S., Latysheva N., Østerud B., Guigner J.M., Sureau F., Bonneau S., Kuzmin A.N., Prasad P.N., et al. Extracellular vesicles from activated platelets possess a phospholipid-rich biomolecular profile and enhance prothrombinase activity. J. Thromb. Haemost. JTH. 2024;22:1463–1474. doi: 10.1016/j.jtha.2024.01.004. - DOI - PubMed
-
- El-Mortada F., Landelouci K., Bertrand-Perron S., Aubé F.A., Poirier A., Bidias A., Jourdi G., Welman M., Gantier M.P., Hamilton J.R., et al. Megakaryocytes possess a STING pathway that is transferred to platelets to potentiate activation. Life Sci. Alliance. 2023;7:e202302211. doi: 10.26508/lsa.202302211. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

