Advances in Melanoma: From Genetic Insights to Therapeutic Innovations
- PMID: 39200315
- PMCID: PMC11351162
- DOI: 10.3390/biomedicines12081851
Advances in Melanoma: From Genetic Insights to Therapeutic Innovations
Abstract
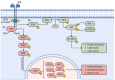
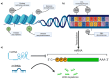
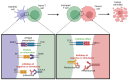
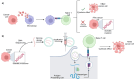
Advances in melanoma research have unveiled critical insights into its genetic and molecular landscape, leading to significant therapeutic innovations. This review explores the intricate interplay between genetic alterations, such as mutations in BRAF, NRAS, and KIT, and melanoma pathogenesis. The MAPK and PI3K/Akt/mTOR signaling pathways are highlighted for their roles in tumor growth and resistance mechanisms. Additionally, this review delves into the impact of epigenetic modifications, including DNA methylation and histone changes, on melanoma progression. The tumor microenvironment, characterized by immune cells, stromal cells, and soluble factors, plays a pivotal role in modulating tumor behavior and treatment responses. Emerging technologies like single-cell sequencing, CRISPR-Cas9, and AI-driven diagnostics are transforming melanoma research, offering precise and personalized approaches to treatment. Immunotherapy, particularly immune checkpoint inhibitors and personalized mRNA vaccines, has revolutionized melanoma therapy by enhancing the body's immune response. Despite these advances, resistance mechanisms remain a challenge, underscoring the need for combined therapies and ongoing research to achieve durable therapeutic responses. This comprehensive overview aims to highlight the current state of melanoma research and the transformative impacts of these advancements on clinical practice.
Keywords: MAPK pathway; PI3K/Akt/mTOR pathway; epigenetic modifications; genetic mutations; immune checkpoint inhibitors; immunotherapy; mRNA vaccines; melanoma; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Future perspectives in melanoma research: meeting report from the "Melanoma Bridge": Napoli, December 3rd-6th 2014.J Transl Med. 2015 Nov 30;13:374. doi: 10.1186/s12967-015-0736-1. J Transl Med. 2015. PMID: 26619946 Free PMC article.
-
Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy.Biomedicines. 2024 Sep 23;12(9):2158. doi: 10.3390/biomedicines12092158. Biomedicines. 2024. PMID: 39335671 Free PMC article. Review.
-
The evolution of BRAF-targeted therapies in melanoma: overcoming hurdles and unleashing novel strategies.Front Oncol. 2024 Nov 8;14:1504142. doi: 10.3389/fonc.2024.1504142. eCollection 2024. Front Oncol. 2024. PMID: 39582535 Free PMC article. Review.
-
Unveiling the genetic and epigenetic landscape of colorectal cancer: new insights into pathogenic pathways.Med Oncol. 2023 Oct 19;40(11):334. doi: 10.1007/s12032-023-02201-8. Med Oncol. 2023. PMID: 37855910 Review.
-
Molecular complexity of diffuse large B-cell lymphoma: a molecular perspective and therapeutic implications.J Appl Genet. 2024 Feb;65(1):57-72. doi: 10.1007/s13353-023-00804-5. Epub 2023 Nov 25. J Appl Genet. 2024. PMID: 38001281 Review.
Cited by
-
Advances in Materials Science for Precision Melanoma Therapy: Nanotechnology-Enhanced Drug Delivery Systems.Pharmaceutics. 2025 Feb 24;17(3):296. doi: 10.3390/pharmaceutics17030296. Pharmaceutics. 2025. PMID: 40142960 Free PMC article. Review.
-
Cancer Stem Cells in Melanoma: Drivers of Tumor Plasticity and Emerging Therapeutic Strategies.Int J Mol Sci. 2025 Aug 1;26(15):7419. doi: 10.3390/ijms26157419. Int J Mol Sci. 2025. PMID: 40806546 Free PMC article. Review.
-
Aggressive nodular melanoma: case report with an unusual BRAF mutation.Mol Biol Rep. 2025 Jul 9;52(1):688. doi: 10.1007/s11033-025-10803-w. Mol Biol Rep. 2025. PMID: 40632324
-
Characterizing CD133 and NANOG Expression in Melanoma: Associations with Histological and Epidemiological Parameters.Medicina (Kaunas). 2024 Oct 10;60(10):1658. doi: 10.3390/medicina60101658. Medicina (Kaunas). 2024. PMID: 39459448 Free PMC article.
-
DNA methylation in melanoma immunotherapy: mechanisms and therapeutic opportunities.Clin Epigenetics. 2025 Apr 30;17(1):71. doi: 10.1186/s13148-025-01865-5. Clin Epigenetics. 2025. PMID: 40307913 Free PMC article. Review.
References
-
- Ferlay J., Ervik M., Lam F., Laversanne M., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., et al. Global Cancer Observatory: Cancer Today Lyon, France: International Agency for Research on Cancer. 2024. [(accessed on 22 June 2024)]. Available online: https://gco.iarc.who.int/today.
-
- Laskar R., Ferreiro-Iglesias A., Bishop D.T., Iles M.M., Kanetsky P.A., Armstrong B.K., Law M.H., Goldstein A.M., Aitken J.F., Giles G.G., et al. Risk factors for melanoma by anatomical site: An evaluation of aetiological heterogeneity. Br. J. Dermatol. 2021;184:1085–1093. doi: 10.1111/bjd.19705. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

