Sensory Neurons Release Cardioprotective Factors in an In Vitro Ischemia Model
- PMID: 39200320
- PMCID: PMC11351881
- DOI: 10.3390/biomedicines12081856
Sensory Neurons Release Cardioprotective Factors in an In Vitro Ischemia Model
Abstract
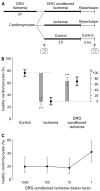
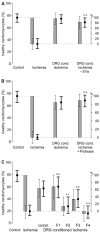
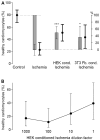
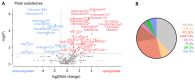
Sensory neurons densely innervate the myocardium. The role of their sensing and response to acute and prolonged ischemia is largely unclear. In a cellular model of ischemia-reperfusion injury, the presence of sensory neurons increases cardiomyocyte survival. Here, after the exclusion of classical neurotransmitter release, and measurement of cytokine release, we modified the experiment from a direct co-culture of primary murine cardiomyocytes and sensory neurons to a transfer of the supernatant. Sensory neurons were exposed to ischemia and the resulting conditioned supernatant was transferred onto cardiomyocytes. This approach largely increased the tolerance of cardiomyocytes to ischemia and reperfusion. Towards the identification of the mechanism, it was demonstrated that after ten-fold dilution, the conditioned solution lost its protective effect. The effect remained after removal of extracellular vesicles by ultracentrifugation, and was not affected by exposure to protease activity, and fractionation pointed towards a hydrophilic agent. Solutions conditioned by HEK293t cells or 3T3 fibroblasts also increase cardiomyocyte survival, but to a lower degree. A metabolomic search identified 64 at least two-fold changed metabolites and lipids. Many of these could be identified and are involved in essential cellular functions. In the presented model for ischemia-reperfusion, sensory neurons secrete one or more cardioprotective substances that can improve cardiomyocyte survival.
Keywords: cardiomyocytes; ischemia; ischemia-reperfusion; sensory neurons.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. - DOI - PubMed
-
- Cowled P., Fitridge R. In: Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists. Fitridge R., Thompson M., editors. University of Adelaide Press; Adelaide, Australia: 2011. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

