The Functional Interaction of KATP and BK Channels with Aquaporin-4 in the U87 Glioblastoma Cell
- PMID: 39200356
- PMCID: PMC11351575
- DOI: 10.3390/biomedicines12081891
The Functional Interaction of KATP and BK Channels with Aquaporin-4 in the U87 Glioblastoma Cell
Abstract
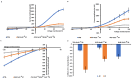
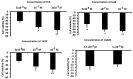
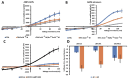
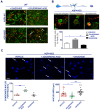
K+ channels do play a role in cell shape changes observed during cell proliferation and apoptosis. Research suggested that the dynamics of the aggregation of Aquaporin-4 (AQP4) into AQP4-OAP isoforms can trigger cell shape changes in malignant glioma cells. Here, we investigated the relationship between AQP4 and some K+ channels in the malignant glioma U87 line. The U87 cells transfected with the human M1-AQP4 and M23-AQP4 isoforms were investigated for morphology, the gene expression of KCNJ8, KCNJ11, ABCC8, ABCC9, KCNMA1, and Cyclin genes by RT-PCR, recording the whole-cell K+ ion currents by patch-clamp experiments. AQP4 aggregation into OAPs increases the plasma membrane functional expression of the Kir6.2 and SUR2 subunits of the KATP channels and of the KCNMA1 of the BK channels in U87 cells leading to a large increase in inward and outward K+ ion currents. These changes were associated with changes in morphology, with a decrease in cell volume in the U87 cells and an increase in the ER density. These U87 cells accumulate in the mitotic and G2 cell cycle. The KATP channel blocker zoledronic acid reduced cell proliferation in both M23 AQP4-OAP and M1 AQP4-tetramer-transfected cells, leading to early and late apoptosis, respectively. The BK channel sustains the efflux of K+ ions associated with the M23 AQP4-OAP expression in the U87 cells, but it is downregulated in the M1 AQP4-tetramer cells. The KATP channels are effective in the M1 AQP4-tetramer and M23 AQP4-OAP cells. Zoledronic acid can be effective in targeting pathogenic M1 AQP4-tetramer cell phenotypes inhibiting KATP channels and inducing early apoptosis.
Keywords: BK channels; KATP channels; M1-AQP4 (AQP4-tetramers forming isoform); M23-AQP4 (AQP4-OAPs forming isoform); aquaporin-4; cell cycle; glioblastoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


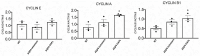
References
-
- Catacuzzeno L., Sforna L., Esposito V., Limatola C., Franciolini F. In: Ion Channels in Glioma Malignancy BT—Transportome Malfunction in the Cancer Spectrum: Ion Transport in Tumor Biology. Stock C., Pardo L.A., editors. Springer International Publishing; Cham, Switzerland: 2021. pp. 223–267.
LinkOut - more resources
Full Text Sources

