Combination of Anti-CD40 and Anti-CD40L Antibodies as Co-Stimulation Blockade in Preclinical Cardiac Xenotransplantation
- PMID: 39200391
- PMCID: PMC11351779
- DOI: 10.3390/biomedicines12081927
Combination of Anti-CD40 and Anti-CD40L Antibodies as Co-Stimulation Blockade in Preclinical Cardiac Xenotransplantation
Abstract
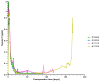
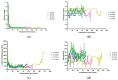
The blockade of the CD40/CD40L immune checkpoint is considered essential for cardiac xenotransplantation. However, it is still unclear which single antibody directed against CD40 or CD40L (CD154), or which combination of antibodies, is better at preventing organ rejection. For example, the high doses of antibody administered in previous experiments might not be feasible for the treatment of humans, while thrombotic side effects were described for first-generation anti-CD40L antibodies. To address these issues, we conducted six orthotopic pig-to-baboon cardiac xenotransplantation experiments, combining a chimeric anti-CD40 antibody with an investigational long-acting PASylated anti-CD40L Fab fragment. The combination therapy effectively resulted in animal survival with a rate comparable to a previous study that utilized anti-CD40 monotherapy. Importantly, no incidence of thromboembolic events associated with the administration of the anti-CD40L PAS-Fab was observed. Two experiments failed early because of technical reasons, two were terminated deliberately after 90 days with the baboons in excellent condition and two were extended to 120 and 170 days, respectively. Unexpectedly, and despite the absence of any clinical signs, histopathology revealed fungal infections in all four recipients. This study provides, for the first time, insights into a combination therapy with anti-CD40/anti-CD40L antibodies to block this immune checkpoint.
Keywords: CD40/CD40L; co-stimulation blockade; heart; orthotopic heart transplantation; xenotransplantation.
Conflict of interest statement
Jan-Michael Abicht, Bruno Reichart, Eckhard Wolf, Paolo Brenner, Arne Skerra and Matthias Längin are founders of XTransplant GmbH. David Ayares is chief executive officer and chief scientific officer of Revivicor, Inc. Michaela Gebauer is an employee, Uli Binder and Arne Skerra are shareholders of XL-protein GmbH. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The other authors declare no conflicts of interest.
Figures






References
-
- Reichart B., Längin M., Radan J., Mokelke M., Buttgereit I., Ying J., Fresch A.K., Mayr T., Issl L., Buchholz S., et al. Pig-to-non-human primate heart transplantation: The final step toward clinical xenotransplantation? J. Heart Lung Transplant. 2020;39:751–757. doi: 10.1016/j.healun.2020.05.004. - DOI - PubMed
-
- Mohiuddin M.M., Goerlich C.E., Singh A.K., Zhang T., Tatarov I., Lewis B., Sentz F., Hershfeld A., Braileanu G., Odonkor P., et al. Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation. 2022;29:e12744. doi: 10.1111/xen.12744. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

