An Analysis of the Intestinal Microbiome Combined with Metabolomics to Explore the Mechanism of How Jasmine Tea Improves Depression in CUMS-Treated Rats
- PMID: 39200564
- PMCID: PMC11353544
- DOI: 10.3390/foods13162636
An Analysis of the Intestinal Microbiome Combined with Metabolomics to Explore the Mechanism of How Jasmine Tea Improves Depression in CUMS-Treated Rats
Abstract
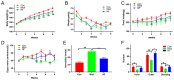
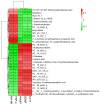
Recently, research has confirmed that jasmine tea may help improve the depressive symptoms that are associated with psychiatric disorders. Our team previously found that jasmine tea improved the depressive-like behavior that is induced by chronic unpredictable mild stress (CUMS) in Sprague Dawley (SD) rats. We hypothesized that the metabolic disorder component of depression may be related to the gut microbiota, which may be reflected in the metabolome in plasma. The influence of jasmine tea on gut microbiota composition and the association with depressive-related indexes were explored. Furthermore, the metabolites in plasma that are related to the gut microbiota were identified. SD rats were treated with control or CUMS and administrated jasmine tea for 8 weeks. The 16S rRNA gene amplicon sequencing was used to analyze the gut microbiota in feces samples, and untargeted metabolomics was used to analyze the metabolites in plasma. The results found that jasmine tea significantly ameliorated the depressive behavior induced by CUMS, significantly improved the neurotransmitter concentration (BDNF and 5-HT), and decreased the pro-inflammation levels (TNF-α and NF-κB). The intervention of jasmine tea also alleviated the dysbiosis caused by CUMS; increased the relative abundance of Bacteroides, Blautia, Clostridium, and Lactobacillus; and decreased Ruminococcus and Butyrivibrio in the CUMS-treated rats. Furthermore, the serum metabolites of the CUMS-treated rats were reversed after the jasmine tea intervention, i.e., 22 were up-regulated and 18 were down-regulated, which may have a close relationship with glycerophospholipid metabolism pathways, glycine serine and threonine metabolism pathways, and nicotinate and nicotinamide metabolism pathways. Finally, there were 30 genera of gut microbiota related to the depressive-related indexes, and 30 metabolites in the plasma had a strong predictive ability for depressive behavior. Potentially, our research implies that the intervention of jasmine tea can ameliorate the depression induced by CUMS via controlling the gut flora and the host's metabolism, which is an innovative approach for the prevention and management of depression.
Keywords: CUMS; gut microbiome; jasmine tea; metabolome.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









References
-
- Kelly J.R., Borre Y., O’Brien C., Patterson E., El Aidy S., Deane J., Kennedy P.J., Beers S., Scott K., Moloney G., et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. - DOI - PubMed
Grants and funding
- CARS-23/National Tea Industry Technology System Project
- 2020NK2026/Innovation and demonstration of key technologies for jasmine tea scenting processing
- 2024JJ7497/The effects and mechanisms of EGCG on reproductive viability in CUMS rats
- Xiangjiaotong [2023] No. 233/Science and Technology Innovation Team support project of Hunan Normal High School
LinkOut - more resources
Full Text Sources

