The Rhinoplasty Outcome Evaluation (ROE) Questionnaire in Rhinoplasty: A Systematic Review and Meta-Analysis
- PMID: 39200785
- PMCID: PMC11355392
- DOI: 10.3390/jcm13164642
The Rhinoplasty Outcome Evaluation (ROE) Questionnaire in Rhinoplasty: A Systematic Review and Meta-Analysis
Abstract
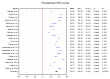
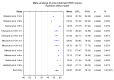
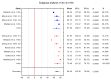
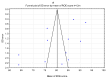
Background/Objectives: This study aims to systematize the ability to use ROE to assess rhinoplasty outcomes in surgical approaches. Methods: The PubMed, Scopus, and Web of Science databases were searched for the following terms: "rhinoplasty and outcome" OR "prognosis" OR "outcomes" OR "satisfaction" OR "quality of life" OR QoL "rhinoplasty outcome evaluation". The timeframe of the included studies is from 2011 to May 2024. Ultimately, 17 papers were included in the conducted meta-analysis of ROE scores between pre- and post-treatment data. Results The mean value of the pre-treatment ROE score was 33.50 with a CI of 29.46 to 37.53 (p < 0.001), while the post-treatment ROE was 69.60 with a CI of 63.07 to 76.14 (t ≤ 6 months). At t = 12 months it was 80.25 with a CI of 75.79 to 84.70 (p < 0.001). The mean difference between pre-treatment and post-treatment scores (t ≤ 6 months) was -36.31 with a CI of -40.93 to -31.69. The mean difference between pre-treatment and post-treatment scores for 6 m < t ≤ 12 m was -47.36 with a CI of -53.89 to -40.83. Conclusions: The result was statistically significant (p < 0.001).
Keywords: ROE; meta-analysis; quality of life; the Rhinoplasty Outcome Evaluation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
A Systematic Review and Meta-Analysis of Rhinoplasty Using the Rhinoplasty Outcome Evaluation Scale.Ann Maxillofac Surg. 2022 Jan-Jun;12(1):60-68. doi: 10.4103/ams.ams_244_21. Epub 2022 Aug 16. Ann Maxillofac Surg. 2022. PMID: 36199467 Free PMC article. Review.
-
Satisfaction in Patients After Rhinoplasty Using the Rhinoplasty Outcome Evaluation Questionnaire.Cureus. 2019 Jul 30;11(7):e5283. doi: 10.7759/cureus.5283. Cureus. 2019. PMID: 31576273 Free PMC article.
-
Quality of life outcome in revision rhinoplasty in regards to number of revision surgeries and cartilage donor site.Auris Nasus Larynx. 2022 Apr;49(2):286-290. doi: 10.1016/j.anl.2021.08.004. Epub 2021 Sep 10. Auris Nasus Larynx. 2022. PMID: 34518029
-
How to predict the outcome of septorhinoplasty? A normative study of ROE and FROI-17 scores.Acta Otorhinolaryngol Ital. 2021 Aug;41(4):327-335. doi: 10.14639/0392-100X-N1291. Acta Otorhinolaryngol Ital. 2021. PMID: 34533536 Free PMC article.
-
Patient Satisfaction following Structural versus Preservation Rhinoplasty: A Systematic Review.Facial Plast Surg. 2020 Oct;36(5):670-678. doi: 10.1055/s-0040-1714268. Epub 2020 Aug 4. Facial Plast Surg. 2020. PMID: 32750725
Cited by
-
Normative Values of Rhinology Questionnaires in Young Adults: A Tool to Identify Candidates for Rhinoplasty.Life (Basel). 2025 Jan 24;15(2):170. doi: 10.3390/life15020170. Life (Basel). 2025. PMID: 40003579 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

