The Manganese-Bone Connection: Investigating the Role of Manganese in Bone Health
- PMID: 39200820
- PMCID: PMC11355939
- DOI: 10.3390/jcm13164679
The Manganese-Bone Connection: Investigating the Role of Manganese in Bone Health
Abstract
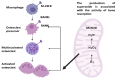
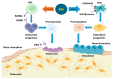
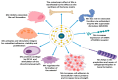
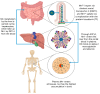
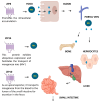
The complex relationship between trace elements and skeletal health has received increasing attention in the scientific community. Among these minerals, manganese (Mn) has emerged as a key element affecting bone metabolism and integrity. This review examines the multifaceted role of Mn in bone health, including its effects on bone regeneration, mineralization, and overall skeletal strength. This review article is based on a synthesis of experimental models, epidemiologic studies, and clinical trials of the mechanisms of the effect of Mn on bone metabolism. Current research data show that Mn is actively involved in the processes of bone remodeling by modulating the activity of osteoblasts and osteoclasts, as well as the main cells that regulate bone formation and resorption. Mn ions have a profound effect on bone mineralization and density by intricately regulating signaling pathways and enzymatic reactions in these cells. Additionally, Mn superoxide dismutase (MnSOD), located in bone mitochondria, plays a crucial role in osteoclast differentiation and function, protecting osteoclasts from oxidative damage. Understanding the nuances of Mn's interaction with bone is essential for optimizing bone strategies, potentially preventing and managing skeletal diseases. Key findings include the stimulation of osteoblast proliferation and differentiation, the inhibition of osteoclastogenesis, and the preservation of bone mass through the RANK/RANKL/OPG pathway. These results underscore the importance of Mn in maintaining bone health and highlight the need for further research into its therapeutic potential.
Keywords: bone; bone health; bone mass; bone metabolism; manganese.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
References
-
- Vanitchanont M., Vallibhakara S.A., Sophonsritsuk A., Vallibhakara O. Effects of Multispecies Probiotic Supplementation on Serum Bone Turnover Markers in Postmenopausal Women with Osteopenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024;16:461. doi: 10.3390/nu16030461. - DOI - PMC - PubMed
-
- Lopes T.S.B., Shi H., White D., Araujo I.C.S., Kim W.K. Effects of 25-hydroxycholecalciferol on performance, gut health, and bone quality of broilers fed with reduced calcium and phosphorus diet during Eimeria challenge. Poult. Sci. 2024;103:103267. doi: 10.1016/j.psj.2023.103267. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

