PRO-NOVELTY: Patient-Reported Outcomes in NOse VEstibule interventionaL radioTherapY (brachytherapy)
- PMID: 39200822
- PMCID: PMC11355133
- DOI: 10.3390/jcm13164683
PRO-NOVELTY: Patient-Reported Outcomes in NOse VEstibule interventionaL radioTherapY (brachytherapy)
Abstract
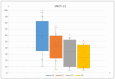
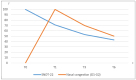
Background: The aim of this paper is to evaluate the impact on the quality of life of the treatment of nasal vestibule tumors by interventional radiotherapy (IRT-brachytherapy) through a patient reported outcome questionnaire. Methods: We prospectively collected data about patients undergoing IRT according to our institutional schedule of 44 Gy delivered in 14 fractions twice a day. We recorded both acute toxicity data, using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, and quality of life data, using the 22-item Sino-Nasal Outcome Test (SNOT-22) at baseline (T0), at 1 month (T1), at 3 months (T3), and at 6 months (T6). Results: We enrolled 10 consecutive patients treated between February 2023 and October 2023. The decrease in terms of SNOT-22 mean value was statistically significant from T0 and T6 with a p-value < 0.001. A noteworthy clinical finding is that quality of life improved regardless of the occurrence of G1-G2 side effects. Conclusions: Using SNOT-22 on patients with nasal vestibule carcinoma treated with IRT has shown an improvement in quality of life that is not strictly dependent on the occurrence of expected G1-G2 side effects.
Keywords: brachytherapy; interventional radiotherapy; nasal vestibule; nose vestibule; quality of life.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- van de Velde L.J., Scheurleer W.F.J., Braunius W.W., Devriese L.A., de Ridder M., de Bree R., Breimer G.E., van Dijk B.A., Rijken J.A. Squamous cell carcinoma of the nasal vestibule in the Netherlands: A clinical and epidemiological review of 763 cases (2008–2021) Head Neck. 2024;46:1809–1821. doi: 10.1002/hed.27749. - DOI - PubMed
-
- Agger A., von Buchwald C., Madsen A.R., Yde J., Lesnikova I., Christensen C.B., Foghsgaard S., Christensen T.B., Hansen H.S., Larsen S., et al. Squamous cell carcinoma of the nasal vestibule 1993-2002: A nationwide retrospective study from DAHANCA. Head Neck. 2009;31:1593–1599. doi: 10.1002/hed.21132. - DOI - PubMed
-
- Filtenborg M.V., Lilja-Fischer J.K., Sharma M.B., Primdahl H., Kjems J., Plaschke C.C., Charabi B.W., Kristensen C.A., Andersen M., Andersen E., et al. Nasal vestibule squamous cell carcinoma: A population-based cohort study from DAHANCA. Acta Oncol. 2022;61:127–133. doi: 10.1080/0284186X.2021.1994646. - DOI - PubMed
LinkOut - more resources
Full Text Sources

