Heart Rate Variability as a Potential Predictor of Response to Intranasal Esketamine in Patients with Treatment-Resistant Depression: A Preliminary Report
- PMID: 39200909
- PMCID: PMC11355362
- DOI: 10.3390/jcm13164767
Heart Rate Variability as a Potential Predictor of Response to Intranasal Esketamine in Patients with Treatment-Resistant Depression: A Preliminary Report
Abstract
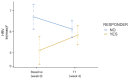
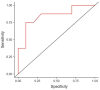
Background: Esketamine has received approval as a nasal spray (ESK-NS) for treatment-resistant depression (TRD) and evidence from real-world investigations has confirmed the effectiveness of ESK-NS, albeit with interindividual differences in response. Heart rate variability (HRV), defined as the fluctuation in time interval between consecutive heartbeats, can be used to measure autonomic dysfunction in psychiatric disorders and its role has been investigated in diagnosis and prognosis of depression. Methods: This preliminary report aims to evaluate HRV parameters and their association with treatment outcome in 18 patients (55.6% males, 55.6 ± 9.39 years old) with TRD treated with a target dose of ESK-NS for one month (mean dose: 80.9 ± 9.05 mg). The Beck Depression Inventory (BDI) and a 3 min resting electrocardiogram were used to assess changes in depressive symptoms and HRV measurements before and after treatment. Results: Responders (n = 8, 44.5%; based on ≥30% BDI scores reduction) displayed lower HRV values than non-responders at baseline (p = 0.019), which increased at one month (p = 0.038). Receiver-Operating Characteristic (ROC) curves obtained from a logistic regression displayed a discriminative potential for baseline HRV in our sample (AUC = 0.844). Conclusions: These preliminary observations suggest a mutual interaction between esketamine and HRV, especially in relation to treatment response. Further studies are required to investigate electrophysiological profiles among predictors of response to ESK-NS and allow for personalized intervention strategies in TRD that still represent a public health concern.
Keywords: biomarkers; esketamine; mood disorders; personalized medicine.
Conflict of interest statement
M.D.N. is/has been a consultant and/or a speaker and/or has received research grants from: Angelini, Janssen, Lundbeck, Neuraxpharm, Otsuka and Idorsia Pharmaceuticals. G.S. is/has been a consultant and/or a speaker and/or has received research grants from: Angelini, Janssen, Lundbeck, Neuraxpharm, and Otsuka.
Figures
Similar articles
-
Investigating the Effectiveness and Tolerability of Intranasal Esketamine Among Older Adults With Treatment-Resistant Depression (TRD): A Post-hoc Analysis from the REAL-ESK Study Group.Am J Geriatr Psychiatry. 2023 Dec;31(12):1032-1041. doi: 10.1016/j.jagp.2023.06.016. Epub 2023 Jul 8. Am J Geriatr Psychiatry. 2023. PMID: 37479669
-
Predicting outcome with Intranasal Esketamine treatment: A machine-learning, three-month study in Treatment-Resistant Depression (ESK-LEARNING).Psychiatry Res. 2023 Sep;327:115378. doi: 10.1016/j.psychres.2023.115378. Epub 2023 Jul 28. Psychiatry Res. 2023. PMID: 37574600
-
Esketamine in treatment-resistant depression patients comorbid with substance-use disorder: A viewpoint on its safety and effectiveness in a subsample of patients from the REAL-ESK study.Eur Neuropsychopharmacol. 2023 Sep;74:15-21. doi: 10.1016/j.euroneuro.2023.04.011. Epub 2023 May 4. Eur Neuropsychopharmacol. 2023. PMID: 37148637
-
An Update on Glutamatergic System in Suicidal Depression and on the Role of Esketamine.Curr Top Med Chem. 2020;20(7):554-584. doi: 10.2174/1568026620666200131100316. Curr Top Med Chem. 2020. PMID: 32003691
-
Exploring Esketamine's Therapeutic Outcomes as an FDA-Designated Breakthrough for Treatment-Resistant Depression and Major Depressive Disorder With Suicidal Intent: A Narrative Review.Cureus. 2024 Feb 10;16(2):e53987. doi: 10.7759/cureus.53987. eCollection 2024 Feb. Cureus. 2024. PMID: 38476783 Free PMC article. Review.
Cited by
-
Cognitive Correlates of Borderline Personality Disorder Features in Youth with Bipolar Spectrum Disorders and Bipolar Offspring.Brain Sci. 2025 Apr 10;15(4):390. doi: 10.3390/brainsci15040390. Brain Sci. 2025. PMID: 40309828 Free PMC article.
-
Understanding Pediatric Bipolar Disorder Through the Investigation of Clinical, Neuroanatomic, Neurophysiological and Neurocognitive Dimensions: A Pilot Study.Brain Sci. 2025 Feb 3;15(2):152. doi: 10.3390/brainsci15020152. Brain Sci. 2025. PMID: 40002485 Free PMC article.
References
-
- Pettorruso M., Guidotti R., D’Andrea G., De Risio L., D’Andrea A., Chiappini S., Carullo R., Barlati S., Zanardi R., REAL-ESK Study Group et al. Predicting outcome with Intranasal Esketamine treatment: A machine-learning, three-month study in Treatment-Resistant Depression (ESK-LEARNING) Psychiatry Res. 2023;327:115378. doi: 10.1016/j.psychres.2023.115378. - DOI - PubMed
-
- Moccia L., Lanzotti P., Pepe M., Palumbo L., Janiri D., Camardese G., Bentivoglio A.R., Di Nicola M., Calabresi P., Sani G. Remission of functional motor symptoms following esketamine administration in a patient with treatment-resistant depression: A single-case report. Int. Clin. Psychopharmacol. 2022;37:21–24. doi: 10.1097/YIC.0000000000000378. - DOI - PubMed
-
- Moccia L., di Luzio M., Conte E., Modica M., Ambrosecchia M., Ardizzi M., Lanzotti P., Kotzalidis G.D., Janiri D., Di Nicola M., et al. Sense of agency and its disturbances: A systematic review targeting the intentional binding effect in neuropsychiatric disorders. Psychiatry Clin. Neurosci. 2024;78:3–18. doi: 10.1111/pcn.13601. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

