Surface Plasmon Resonance Immunosensor for Direct Detection of Antibodies against SARS-CoV-2 Nucleocapsid Protein
- PMID: 39201259
- PMCID: PMC11354133
- DOI: 10.3390/ijms25168574
Surface Plasmon Resonance Immunosensor for Direct Detection of Antibodies against SARS-CoV-2 Nucleocapsid Protein
Abstract
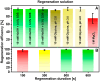
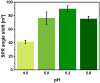
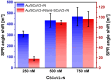
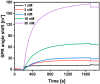
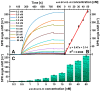
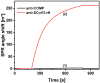
The strong immunogenicity of the SARS-CoV-2 nucleocapsid protein is widely recognized, and the detection of specific antibodies is critical for COVID-19 diagnostics in patients. This research proposed direct, label-free, and sensitive detection of antibodies against the SARS-CoV-2 nucleocapsid protein (anti-SCoV2-rN). Recombinant SARS-CoV-2 nucleocapsid protein (SCoV2-rN) was immobilized by carbodiimide chemistry on an SPR sensor chip coated with a self-assembled monolayer of 11-mercaptoundecanoic acid. When immobilized under optimal conditions, a SCoV2-rN surface mass concentration of 3.61 ± 0.52 ng/mm2 was achieved, maximizing the effectiveness of the immunosensor for the anti-SCoV2-rN determination. The calculated KD value of 6.49 × 10-8 ± 5.3 × 10-9 M confirmed the good affinity of the used monoclonal anti-SCoV2-rN antibodies. The linear range of the developed immunosensor was from 0.5 to 50 nM of anti-SCoV2-rN, where the limit of detection and the limit of quantification values were 0.057 and 0.19 nM, respectively. The immunosensor exhibited good reproducibility and specificity. In addition, the developed immunosensor is suitable for multiple anti-SCoV2-rN antibody detections.
Keywords: SARS-CoV-2 nucleocapsid protein; antibody; immunosensor; surface plasmon resonance.
Conflict of interest statement
The authors report to have no potential conflicts of interest related to this article.
Figures
References
-
- Szekely J., Mongkolprasert J., Jeayodae N., Senorit C., Chaimuti P., Swangphon P., Nanakorn N., Nualnoi T., Wongwitwichot P., Pengsakul T. Development, Analytical, and Clinical Evaluation of Rapid Immunochromatographic Antigen Test for SARS-CoV-2 Variants Detection. Diagnostics. 2022;12:381. doi: 10.3390/diagnostics12020381. - DOI - PMC - PubMed
-
- World Health Organization WHO COVID-19 Dashboard, World Health Organization. 2022. [(accessed on 18 July 2024)]. Available online: https://data.who.int/dashboards/covid19/cases?n=o.
-
- Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., He S., Zhou Z., Zhou Z., Chen Q., et al. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B. 2020;10:1228–1238. doi: 10.1016/j.apsb.2020.04.009. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

