Modulation of Ceramide-Induced Apoptosis in Enteric Neurons by Aryl Hydrocarbon Receptor Signaling: Unveiling a New Pathway beyond ER Stress
- PMID: 39201268
- PMCID: PMC11354200
- DOI: 10.3390/ijms25168581
Modulation of Ceramide-Induced Apoptosis in Enteric Neurons by Aryl Hydrocarbon Receptor Signaling: Unveiling a New Pathway beyond ER Stress
Abstract
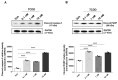
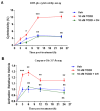
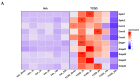
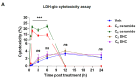
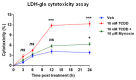
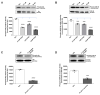
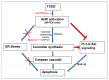
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a persistent organic pollutant and a potent aryl hydrocarbon receptor (AHR) ligand, causes delayed intestinal motility and affects the survival of enteric neurons. In this study, we investigated the specific signaling pathways and molecular targets involved in TCDD-induced enteric neurotoxicity. Immortalized fetal enteric neuronal (IM-FEN) cells treated with 10 nM TCDD exhibited cytotoxicity and caspase 3/7 activation, indicating apoptosis. Increased cleaved caspase-3 expression with TCDD treatment, as assessed by immunostaining in enteric neuronal cells isolated from WT mice but not in neural crest cell-specific Ahr deletion mutant mice (Wnt1Cre+/-/Ahrb(fl/fl)), emphasized the pivotal role of AHR in this process. Importantly, the apoptosis in IM-FEN cells treated with TCDD was mediated through a ceramide-dependent pathway, independent of endoplasmic reticulum stress, as evidenced by increased ceramide synthesis and the reversal of cytotoxic effects with myriocin, a potent inhibitor of ceramide biosynthesis. We identified Sptlc2 and Smpd2 as potential gene targets of AHR in ceramide regulation by a chromatin immunoprecipitation (ChIP) assay in IM-FEN cells. Additionally, TCDD downregulated phosphorylated Akt and phosphorylated Ser9-GSK-3β levels, implicating the PI3 kinase/AKT pathway in TCDD-induced neurotoxicity. Overall, this study provides important insights into the mechanisms underlying TCDD-induced enteric neurotoxicity and identifies potential targets for the development of therapeutic interventions.
Keywords: AHR; ENS; TCDD; apoptosis; ceramides; cytotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Van Den Berg M., Birnbaum L.S., Denison M., De Vito M., Farland W., Feeley M., Fiedler H., Håkansson H., Hanberg A., Haws L., et al. The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds. Toxicol. Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R35 ES035027/ES/NIEHS NIH HHS/United States
- 028244/ES/NIEHS NIH HHS/United States
- R35 ES028244/ES/NIEHS NIH HHS/United States
- This work is/was supported by the USDA National Institute of Food and Agriculture and Hatch Appropriations under Project #PEN04917 and Accession #7006412./USDA
- 035027/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

