Control of Dopamine Signal in High-Order Receptor Complex on Striatal Astrocytes
- PMID: 39201299
- PMCID: PMC11354247
- DOI: 10.3390/ijms25168610
Control of Dopamine Signal in High-Order Receptor Complex on Striatal Astrocytes
Abstract
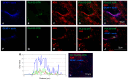
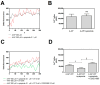
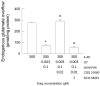
The receptor-receptor interaction (RRI) of G protein-coupled receptors (GPCRs) leads to new functional entities that are conceptually distinct from the simple addition of signals mediated by the activation of the receptors that form the heteromers. Focusing on astrocytes, there is evidence for the existence of inhibitory and facilitatory RRIs, including the heteromers formed by the adenosine A2A and the dopamine D2 receptors, by A2A and the oxytocin receptor (OTR), and the D2-OTR heteromers. The possible involvement of these receptors in mosaicism has never been investigated in striatal astrocytes. By biophysical and functional approaches, we focused our attention on the existence of an A2A-D2-OTR high-order receptor complex and its role in modulating cytosolic calcium levels and endogenous glutamate release, when striatal astrocyte processes were stimulated with 4-aminopyridine. Functional data indicate a permissive role of OTR on dopamine signaling in the regulation of the glutamatergic transmission, and an inhibitory control mediated by A2A on both the D2-mediated signaling and on the OTR-facilitating effect on D2. Imaging biochemical and bioinformatic evidence confirmed the existence of the A2A-D2-OTR complex and its ternary structure in the membrane. In conclusion, the D2 receptor appears to be a hotspot in the control of the glutamate release from the astrocytic processes and may contribute to the regulation and integration of different neurotransmitter-mediated signaling in the striatum by the A2A-D2-OTR heterotrimers. Considering the possible selectivity of allosteric interventions on GPCRs organized as receptor mosaics, A2A-D2-OTR heterotrimers may offer selective pharmacological targets in neuropsychiatric disorders and neurodegenerative diseases.
Keywords: adenosine receptor; astrocyte process; dopamine receptor; glutamate; heteromers; high-order receptor complex; intracellular calcium; neuroglia; oxytocin; receptor mosaic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



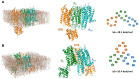
References
-
- Agnati L.F., Fuxe K., Zini I., Lenzi P., Hökfelt T. Aspects on receptor regulation and isoreceptor identification. Med. Biol. 1980;58:182–187. - PubMed
-
- Fuxe K., Agnati L.F., Benfenati F., Celani M., Zini I., Zoli M., Zini I., Zoli M., Mutt V. Evidence for the existence of receptor-receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. J. Neural Transm. 1983;18:165–179. - PubMed
-
- Agnati L.F., Fuxe K., Giardino L., Calzà L., Zoli M., Battistini N., Benfenati F., Vanderhaeghen J.J., Guidolin D., Ruggeri M. Evidence for cholecystokinin-dopamine receptor interactions in the central nervous system of the adult and old rat. Studies on their functional meaning. Ann. N. Y. Acad. Sci. 1985;448:315–333. doi: 10.1111/j.1749-6632.1985.tb29927.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- FFABR to CC and MM/Italian Ministry of University and Research
- Contribution 2021 to S.A and contribution 2023 to E.F/Ph.D. School of the Department of Experimental Medicine (University of Genova)
- project MNESYS (PE0000006) - A Mul-tiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022)/#NEXTGENERATIONEU (NGEU) and funded by the Italian Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP),
- RBAP11ETKA-005 to DIFILAB/Italian Ministry of University and Research
LinkOut - more resources
Full Text Sources

