Metabolomic Profiling of Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy for Predicting Disease-Free and Overall Survival
- PMID: 39201325
- PMCID: PMC11354796
- DOI: 10.3390/ijms25168639
Metabolomic Profiling of Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy for Predicting Disease-Free and Overall Survival
Abstract
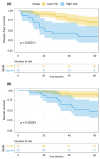
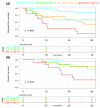
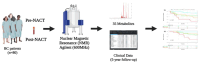
Breast cancer (BC) remains a significant global health concern, with neoadjuvant chemotherapy (NACT) offering preoperative benefits like tumor downstaging and treatment response assessment. However, identifying factors influencing post-NACT treatment response and survival outcomes is challenging. Metabolomic approaches offer promising insights into understanding these outcomes. This study analyzed the serum of 80 BC patients before and after NACT, followed for up to five years, correlating with disease-free survival (DFS) and overall survival (OS). Using untargeted nuclear magnetic resonance (NMR) spectroscopy and a novel statistical model that avoids collinearity issues, we identified metabolic changes associated with survival outcomes. Four metabolites (histidine, lactate, serine, and taurine) were significantly associated with DFS. We developed a metabolite-related survival score (MRSS) from these metabolites, stratifying patients into low- and high-risk relapse groups, independent of classical prognostic factors. High-risk patients had a hazard ratio (HR) for DFS of 3.42 (95% CI 1.51-7.74; p = 0.003) after adjustment for disease stage and age. A similar trend was observed for OS (HR of 3.34, 95% CI 1.64-6.80; p < 0.001). Multivariate Cox proportional hazards analysis confirmed the independent prognostic value of the MRSS. Our findings suggest the potential of metabolomic data, alongside traditional markers, in guiding personalized treatment decisions and risk stratification in BC patients undergoing NACT. This study provides a methodological framework for leveraging metabolomics in survival analyses.
Keywords: NMR spectroscopy; mammary cancer; metabolome; neoadjuvant chemotherapy; survival analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Denkert C., von Minckwitz G., Darb-Esfahani S., Lederer B., Heppner B.I., Weber K.E., Budczies J., Huober J., Klauschen F., Furlanetto J., et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: A Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. Lancet. Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. - DOI - PubMed
-
- Debik J., Euceda L.R., Lundgren S., Gythfeldt H.V.D.L., Garred Ø., Borgen E., Engebraaten O., Bathen T.F., Giskeødegård G.F. Assessing Treatment Response and Prognosis by Serum and Tissue Metabolomics in Breast Cancer Patients. J. Proteome Res. 2019;18:3649–3660. doi: 10.1021/acs.jproteome.9b00316. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

