Modifying Membranotropic Action of Antimicrobial Peptide Gramicidin S by Star-like Polyacrylamide and Lipid Composition of Nanocontainers
- PMID: 39201384
- PMCID: PMC11354511
- DOI: 10.3390/ijms25168691
Modifying Membranotropic Action of Antimicrobial Peptide Gramicidin S by Star-like Polyacrylamide and Lipid Composition of Nanocontainers
Abstract
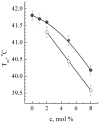
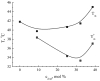
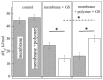
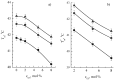
Gramicidin S (GS), one of the first discovered antimicrobial peptides, still shows strong antibiotic activity after decades of clinical use, with no evidence of resistance. The relatively high hemolytic activity and narrow therapeutic window of GS limit its use in topical applications. Encapsulation and targeted delivery may be the way to develop the internal administration of this drug. The lipid composition of membranes and non-covalent interactions affect GS's affinity for and partitioning into lipid bilayers as monomers or oligomers, which are crucial for GS activity. Using both differential scanning calorimetry (DSC) and FTIR methods, the impact of GS on dipalmitoylphosphatidylcholine (DPPC) membranes was tested. Additionally, the combined effect of GS and cholesterol on membrane characteristics was observed; while dipalmitoylphosphatydylglycerol (DPPG) and cerebrosides did not affect GS binding to DPPC membranes, cholesterol significantly altered the membrane, with 30% mol concentration being most effective in enhancing GS binding. The effect of star-like dextran-polyacrylamide D-g-PAA(PE) on GS binding to the membrane was tested, revealing that it interacted with GS in the membrane and significantly increased the proportion of GS oligomers. Instead, calcium ions affected GS binding to the membrane differently, with independent binding of calcium and GS and no interaction between them. This study shows how GS interactions with lipid membranes can be effectively modulated, potentially leading to new formulations for internal GS administration. Modified liposomes or polymer nanocarriers for targeted GS delivery could be used to treat protein misfolding disorders and inflammatory conditions associated with free-radical processes in cell membranes.
Keywords: Fourier transform infrared spectroscopy FTIR; antimicrobial peptide; differential scanning calorimetry DSC; drug delivery; gramicidin S; lipid membranes; nanocontainers.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Babii O., Afonin S., Ishchenko A.Y., Schober T., Negelia A.O., Tolstanova G.M., Garmanchuk L.V., Ostapchenko L.I., Komarov I.V., Ulrich A.S. Structure-Activity Relationships of Photoswitchable Diarylethene-Based β-Hairpin Peptides as Membranolytic Antimicrobial and Anticancer Agents. J. Med. Chem. 2018;61:10793–10813. doi: 10.1021/acs.jmedchem.8b01428. - DOI - PubMed
-
- Prenner E.J., Lewis R.N., Kondejewski L.H., Hodges R.S., McElhaney R.N. Differential scanning calorimetric study of the effect of the antimicrobial peptide gramicidin S on the thermotropic phase behavior of phosphatidylcholine, phosphatidylethanolamine and phosphatidylglycerol lipid bilayer membranes. Biochim. Biophys. Acta. 1999;1417:211–223. doi: 10.1016/S0005-2736(99)00004-8. - DOI - PubMed
-
- Toyran N., Severcan F. Infrared Spectroscopic Studies on the Dipalmitoyl Phosphatidylcholine Bilayer Interactions with Calcium Phosphate: Effect of Vitamin D2. J. Spectrosc. 2002;16:381692. doi: 10.1155/2002/381692. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

