Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice
- PMID: 39201385
- PMCID: PMC11354499
- DOI: 10.3390/ijms25168701
Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice
Abstract
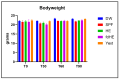
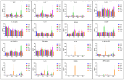
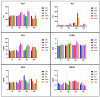
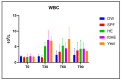
Three hyperimmune egg-based formulations rich in immunoglobulin Y (IgY) were orally administered (daily, for up to 90 days) to C57BL/6 mice that were not microbially challenged. The serum levels of 32 cytokines were quantified every 30 days. Histopathology, hematology, and serum biochemistry investigations were also performed. As a sign of increased immune activity, lymphohistiocytic infiltrates were detected in the digestive tract and the liver after 30, 60, and 90 days of treatment. These infiltrates were also present in the lungs after 30 and 60 days, but not at 90 days. Blood analysis indicated systemic inflammation after 30 days of treatment: increases in pro-inflammatory cytokines, glycemia, total serum proteins, ALT, and ALP. After 60 and 90 days of treatment, the analyzed blood parameters showed mixed signs of both increased and decreased inflammation. The increased cytokines, which varied with formulation and time of exposure, indicated a combination of mostly Th17- and Th2-type immune responses. As the mice were healthy and housed in standardized sanitary conditions, and were not microbially challenged, the data were consistent with an interaction of IgY with the gut-associated lymphoid tissue as the main mechanism of action. This interaction generated a local immune response, which subsequently induced a systemic response.
Keywords: C57BL/6 mice; biochemistry; cytokines; hematology; histopathology; hyperimmune egg; immune response; immunoglobulin Y; interleukins; oral administration.
Conflict of interest statement
The authors declare that this study received funding from the company SC Romvac SA. The funder had the following involvement with this study: the company provided the tested products and partial financing for the materials necessary for the research. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. Authors Alina-Elena Stefan and Daniela Gologan were employed by the company Themis Pathology SRL. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Elevated T-helper 2 cytokine levels in high fat diet-fed C57BL/6 mice are attenuated by short-term 6-week treatment with a combination of low-dose aspirin and metformin.Cytokine. 2020 Apr;128:154999. doi: 10.1016/j.cyto.2020.154999. Epub 2020 Jan 31. Cytokine. 2020. PMID: 32014718
-
Oral vaccination with influenza hemagglutinin combined with human pulmonary surfactant-mimicking synthetic adjuvant SF-10 induces efficient local and systemic immunity compared with nasal and subcutaneous vaccination and provides protective immunity in mice.Vaccine. 2019 Jan 21;37(4):612-622. doi: 10.1016/j.vaccine.2018.12.002. Epub 2018 Dec 13. Vaccine. 2019. PMID: 30553569
-
Immunomodulation of TH2 biased immunity with mucosal administration of nanoemulsion adjuvant.Vaccine. 2016 Jul 25;34(34):4017-24. doi: 10.1016/j.vaccine.2016.06.043. Epub 2016 Jun 23. Vaccine. 2016. PMID: 27317451 Free PMC article.
-
The C-type Lectin Receptor-Driven, Th17 Cell-Mediated Severe Pathology in Schistosomiasis: Not All Immune Responses to Helminth Parasites Are Th2 Dominated.Front Immunol. 2019 Jan 30;10:26. doi: 10.3389/fimmu.2019.00026. eCollection 2019. Front Immunol. 2019. PMID: 30761125 Free PMC article. Review.
-
The common mucosal immune system: from basic principles to enteric vaccines with relevance for the female reproductive tract.Reprod Fertil Dev. 1994;6(3):369-79. doi: 10.1071/rd9940369. Reprod Fertil Dev. 1994. PMID: 7831485 Review.
Cited by
-
Latest Findings in Immunoglobulin Y Technologies and Applications.Int J Mol Sci. 2025 Jul 2;26(13):6380. doi: 10.3390/ijms26136380. Int J Mol Sci. 2025. PMID: 40650158 Free PMC article. Review.
References
-
- Dean K.L. Hyperimmune Eggs Capture Natural Immune Support. Altern. Complement. Ther. 2000;6:118–124. doi: 10.1089/act.2000.6.118. - DOI
-
- Yokoyama H., Peralta R.C., Umeda K., Hashi T., Icatlo F.C., Kuroki M., Ikemori Y., Kodama Y. Prevention of Fatal Salmonellosis in Neonatal Calves, Using Orally Administered Chicken Egg Yolk Salmonella-Specific Antibodies. Am. J. Vet. Res. 1998;59:416–420. doi: 10.2460/ajvr.1998.59.04.416. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

