Hallmarks for Thrombotic and Hemorrhagic Risks in Chronic Kidney Disease Patients
- PMID: 39201390
- PMCID: PMC11354877
- DOI: 10.3390/ijms25168705
Hallmarks for Thrombotic and Hemorrhagic Risks in Chronic Kidney Disease Patients
Abstract
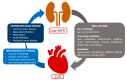
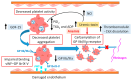
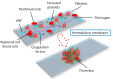
Chronic kidney disease (CKD) is a global health issue causing a significant health burden. CKD patients develop thrombotic and hemorrhagic complications, and cardiovascular diseases are associated with increased hospitalization and mortality in this population. The hemostatic alterations are multifactorial in these patients; therefore, the results of different studies are varying and controversial. Endothelial and platelet dysfunction, coagulation abnormalities, comorbidities, and hemoincompatibility of the dialysis membranes are major contributors of hypo- and hypercoagulability in CKD patients. Due to the tendency of CKD patients to exhibit a prothrombotic state and bleeding risk, they require personalized clinical assessment to understand the impact of antithrombotic therapy. The evidence of efficacy and safety of antiplatelet and anticoagulant treatments is limited for end-stage renal disease patients due to their exclusion from major randomized clinical trials. Moreover, designing hemocompatible dialyzer membranes could be a suitable approach to reduce platelet activation, coagulopathy, and thrombus formation. This review discusses the molecular mechanisms underlying thrombotic and hemorrhagic risk in patients with CKD, leading to cardiovascular complications in these patients, as well as the evidence and guidance for promising approaches to optimal therapeutic management.
Keywords: anticoagulants; antiplatelets; chronic kidney disease; hemodialysis membranes; hemostatic dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Management of patients with atrial fibrillation and chronic kidney disease in light of the latest guidelines.Pol Arch Med Wewn. 2016 May 31;126(5):353-62. doi: 10.20452/pamw.3431. Epub 2016 May 31. Pol Arch Med Wewn. 2016. PMID: 27243343 Review.
-
Stroke prevention in atrial fibrillation patients with chronic kidney disease.Can J Cardiol. 2013 Jul;29(7 Suppl):S71-8. doi: 10.1016/j.cjca.2013.04.005. Can J Cardiol. 2013. PMID: 23790601 Review.
-
Global coagulation assays in patients with chronic kidney disease and their role in predicting thrombotic risk.Thromb Res. 2023 Jun;226:127-135. doi: 10.1016/j.thromres.2023.04.016. Epub 2023 Apr 24. Thromb Res. 2023. PMID: 37150026
-
Platelet Abnormalities in CKD and Their Implications for Antiplatelet Therapy.Clin J Am Soc Nephrol. 2022 Jan;17(1):155-170. doi: 10.2215/CJN.04100321. Epub 2021 Nov 8. Clin J Am Soc Nephrol. 2022. PMID: 34750169 Free PMC article. Review.
-
[T2HD Study. Oral anticoagulants and antiplatelet agents: Practices, benefits, and risks in the chronic hemodialysis population. Observational data].Nephrol Ther. 2016 Jun;12(3):156-65. doi: 10.1016/j.nephro.2015.08.005. Epub 2015 Nov 27. Nephrol Ther. 2016. PMID: 26631311 French.
Cited by
-
The Association of Histological Signs of Plaque Instability with Low eGFR, Higher Neutrophil-to-Lymphocyte Ratio, and Lower Serum MCP-1 Levels in Carotid Endarterectomy Patients-A Single-Center, Prospective Cohort Study.Life (Basel). 2025 Jun 25;15(7):1008. doi: 10.3390/life15071008. Life (Basel). 2025. PMID: 40724511 Free PMC article.
References
-
- Levin A., Stevens P.E., Bilous R.W., Coresh J., Francisco A.L.M.D., Jong P.E.D., Griffith K.E., Hemmelgarn B.R., Iseki K., Lamb E.J., et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. - DOI
-
- Stevens P.E., Ahmed S.B., Carrero J.J., Foster B., Francis A., Hall R.K., Herrington W.G., Hill G., Inker L.A., Kazancıoğlu R., et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105:S117–S314. doi: 10.1016/j.kint.2023.10.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

