Astaxanthin, Compared to Other Carotenoids, Increases the Efficacy of Methotrexate in Rat Adjuvant Arthritis
- PMID: 39201397
- PMCID: PMC11354740
- DOI: 10.3390/ijms25168710
Astaxanthin, Compared to Other Carotenoids, Increases the Efficacy of Methotrexate in Rat Adjuvant Arthritis
Abstract
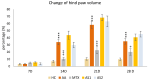
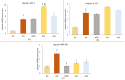
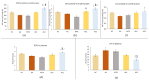
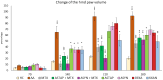
This in vivo study performed in rat adjuvant arthritis aims to advance the understanding of astaxanthin's therapeutic properties for the possible treatment of rheumatoid arthritis (RA) in monotherapy and along with the standard RA treatment, methotrexate (MTX), in combination therapy. The main goal was to elucidate astaxanthin's full therapeutic potential, evaluate its dose dependency, and compare its effects in monotherapy with other carotenoids such as β-carotene and β-cryptoxanthin (KXAN). Moreover, potential differences in therapeutic activity caused by using different sources of astaxanthin, synthetic (ASYN) versus isolated from Blakeslea trispora (ASTAP), were evaluated using one-way ANOVA (Tukey-Kramer post hoc test). KXAN was the most effective in reducing plasma MMP-9 levels in monotherapy, significantly better than MTX, and in reducing hind paw swelling. The differences in the action of ASTAP and ASYN have been observed across various biometric, anti-inflammatory, and antioxidative parameters. In combined therapy with MTX, the ASYN + MTX combination proved to be better. These findings, especially the significant anti-arthritic effect of KXAN and ASYN + MTX, could be the basis for further preclinical studies.
Keywords: adjuvant arthritis; astaxanthin; beta-carotene; beta-cryptoxanthin; carotenoids; inflammation; methotrexate; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Gupta A.K., Seth K., Maheshwari K., Baroliya P.K., Meena M., Kumar A., Vinayak V., Harish Biosynthesis and extraction of high-value carotenoid from algae. Front. Biosci. 2021;26:171–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

