Microbiota-Derived Extracellular Vesicle as Emerging Actors in Host Interactions
- PMID: 39201409
- PMCID: PMC11354844
- DOI: 10.3390/ijms25168722
Microbiota-Derived Extracellular Vesicle as Emerging Actors in Host Interactions
Abstract
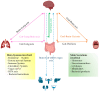
The human microbiota is an intricate micro-ecosystem comprising a diverse range of dynamic microbial populations mainly consisting of bacteria, whose interactions with hosts strongly affect several physiological and pathological processes. The gut microbiota is being increasingly recognized as a critical player in maintaining homeostasis, contributing to the main functions of the intestine and distal organs such as the brain. However, gut dysbiosis, characterized by composition and function alterations of microbiota with intestinal barrier dysfunction has been linked to the development and progression of several pathologies, including intestinal inflammatory diseases, systemic autoimmune diseases, such as rheumatic arthritis, and neurodegenerative diseases, such as Alzheimer's disease. Moreover, oral microbiota research has gained significant interest in recent years due to its potential impact on overall health. Emerging evidence on the role of microbiota-host interactions in health and disease has triggered a marked interest on the functional role of bacterial extracellular vesicles (BEVs) as mediators of inter-kingdom communication. Accumulating evidence reveals that BEVs mediate host interactions by transporting and delivering into host cells effector molecules that modulate host signaling pathways and cell processes, influencing health and disease. This review discusses the critical role of BEVs from the gut, lung, skin and oral cavity in the epithelium, immune system, and CNS interactions.
Keywords: bacterial extracellular vesicles; dysbiosis; gut-brain axis; immune-cell-response; microbiota; neuroninflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wang B., Yao M., Lv L., Ling Z., Li L. The human microbiota in health and disease. Engineering. 2017;3:71–82. doi: 10.1016/J.ENG.2017.01.008. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

