Beyond Anticoagulation: A Comprehensive Review of Non-Vitamin K Oral Anticoagulants (NOACs) in Inflammation and Protease-Activated Receptor Signaling
- PMID: 39201414
- PMCID: PMC11355043
- DOI: 10.3390/ijms25168727
Beyond Anticoagulation: A Comprehensive Review of Non-Vitamin K Oral Anticoagulants (NOACs) in Inflammation and Protease-Activated Receptor Signaling
Abstract
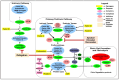
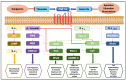
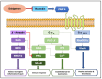
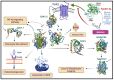
Non-vitamin K oral anticoagulants (NOACs) have revolutionized anticoagulant therapy, offering improved safety and efficacy over traditional agents like warfarin. This review comprehensively examines the dual roles of NOACs-apixaban, rivaroxaban, edoxaban, and dabigatran-not only as anticoagulants, but also as modulators of inflammation via protease-activated receptor (PAR) signaling. We highlight the unique pharmacotherapeutic properties of each NOAC, supported by key clinical trials demonstrating their effectiveness in preventing thromboembolic events. Beyond their established anticoagulant roles, emerging research suggests that NOACs influence inflammation through PAR signaling pathways, implicating factors such as factor Xa (FXa) and thrombin in the modulation of inflammatory responses. This review synthesizes current evidence on the anti-inflammatory potential of NOACs, exploring their impact on inflammatory markers and conditions like atherosclerosis and diabetes. By delineating the mechanisms by which NOACs mediate anti-inflammatory effects, this work aims to expand their therapeutic utility, offering new perspectives for managing inflammatory diseases. Our findings underscore the broader clinical implications of NOACs, advocating for their consideration in therapeutic strategies aimed at addressing inflammation-related pathologies. This comprehensive synthesis not only enhances understanding of NOACs' multifaceted roles, but also paves the way for future research and clinical applications in inflammation and cardiovascular health.
Keywords: NOAC; anti-inflammation; factor Xa; protease-activated-receptor signaling; thrombin.
Conflict of interest statement
The authors declare that they have no competing financial interests or personal relationships which could have influenced the work reported in this paper.
Figures

Similar articles
-
Prescribing of NOACs has outnumbered warfarin: exploring how physicians choose anticoagulant treatments.Eur J Clin Pharmacol. 2018 Mar;74(3):323-330. doi: 10.1007/s00228-017-2374-4. Epub 2017 Nov 17. Eur J Clin Pharmacol. 2018. PMID: 29149366
-
Analysis of Recurrent Stroke Volume and Prognosis between Warfarin and Four Non-Vitamin K Antagonist Oral Anticoagulants' Administration for Secondary Prevention of Stroke.J Stroke Cerebrovasc Dis. 2018 Feb;27(2):338-345. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.007. Epub 2017 Oct 13. J Stroke Cerebrovasc Dis. 2018. PMID: 29033229
-
Practical recommendations on incorporating new oral anticoagulants into routine practice.Clin Adv Hematol Oncol. 2014 Oct;12(10):675-83. Clin Adv Hematol Oncol. 2014. PMID: 25658892 Review.
-
Adherence to Rivaroxaban, Dabigatran, and Apixaban for Stroke Prevention in Incident, Treatment-Naïve Nonvalvular Atrial Fibrillation.J Manag Care Spec Pharm. 2016 Nov;22(11):1319-1329. doi: 10.18553/jmcp.2016.22.11.1319. J Manag Care Spec Pharm. 2016. PMID: 27783556 Free PMC article.
-
Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies.Eur J Epidemiol. 2019 Feb;34(2):173-190. doi: 10.1007/s10654-018-0415-7. Epub 2018 Jun 8. Eur J Epidemiol. 2019. PMID: 29948370
Cited by
-
Integrated Care in Atrial Fibrillation: A Multidisciplinary Approach to Improve Clinical Outcomes and Quality of Life.Healthcare (Basel). 2025 Feb 5;13(3):325. doi: 10.3390/healthcare13030325. Healthcare (Basel). 2025. PMID: 39942514 Free PMC article. Review.
-
Preparation of Carrier-Free Inhalable Dry Powder of Rivaroxaban Using Two-Step Milling for Lung-Targeted Delivery.Pharmaceutics. 2025 May 9;17(5):634. doi: 10.3390/pharmaceutics17050634. Pharmaceutics. 2025. PMID: 40430925 Free PMC article.
-
Selective Modulation of PAR-2-Driven Inflammatory Pathways by Oleocanthal: Attenuation of TNF-α and Calcium Dysregulation in Colorectal Cancer Models.Int J Mol Sci. 2025 Mar 24;26(7):2934. doi: 10.3390/ijms26072934. Int J Mol Sci. 2025. PMID: 40243559 Free PMC article.
-
Lower CALLY index levels indicate higher poor functional outcome risk in acute ischemic stroke patients treated with endovascular thrombectomy.Front Aging Neurosci. 2025 Apr 25;17:1587861. doi: 10.3389/fnagi.2025.1587861. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40353064 Free PMC article.
-
Oleocanthal as a Multifunctional Anti-Cancer Agent: Mechanistic Insights, Advanced Delivery Strategies, and Synergies for Precision Oncology.Int J Mol Sci. 2025 Jun 9;26(12):5521. doi: 10.3390/ijms26125521. Int J Mol Sci. 2025. PMID: 40564985 Free PMC article. Review.
References
-
- England N.H.S.N. Community Pharmacy Oral Anticoagulant Safety Audit 2021/22: NHS England. 2023. [(accessed on 15 June 2024)]. Available online: https://www.england.nhs.uk/long-read/community-pharmacy-oral-anticoagula....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

