Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches
- PMID: 39201418
- PMCID: PMC11354927
- DOI: 10.3390/ijms25168731
Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches
Abstract
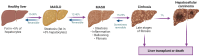
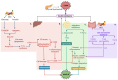
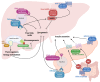
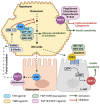
Type 2 diabetes mellitus (T2DM), often featuring hyperglycemia or insulin resistance, is a global health concern that is increasing in prevalence in the United States and worldwide. A common complication is metabolic dysfunction-associated steatotic liver disease (MASLD), the hepatic manifestation of metabolic syndrome that is also rapidly increasing in prevalence. The majority of patients with T2DM will experience MASLD, and likewise, individuals with MASLD are at an increased risk for developing T2DM. These two disorders may act synergistically, in part due to increased lipotoxicity and inflammation within the liver, among other causes. However, the pathophysiological mechanisms by which this occurs are unclear, as is how the improvement of one disorder can ameliorate the other. This review aims to discuss the pathogenic interactions between T2D and MASLD, and will highlight novel therapeutic targets and ongoing clinical trials for the treatment of these diseases.
Keywords: MASLD; hyperglycemia; liver disease; metabolic disease; steatosis.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Ma R.C.W., Tong P.C.Y. Textbook of Diabetes. Wiley; Hoboken, NJ, USA: 2024. Epidemiology of Type 2 Diabetes; pp. 55–74.
-
- Ajmera V., Cepin S., Tesfai K., Hofflich H., Cadman K., Lopez S., Madamba E., Bettencourt R., Richards L., Behling C., et al. A Prospective Study on the Prevalence of NAFLD, Advanced Fibrosis, Cirrhosis and Hepatocellular Carcinoma in People with Type 2 Diabetes. J. Hepatol. 2023;78:471–478. doi: 10.1016/j.jhep.2022.11.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

