Recombinant Subunit Vaccine Candidate against the Bovine Viral Diarrhea Virus
- PMID: 39201420
- PMCID: PMC11354329
- DOI: 10.3390/ijms25168734
Recombinant Subunit Vaccine Candidate against the Bovine Viral Diarrhea Virus
Abstract
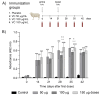
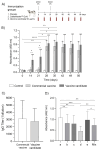
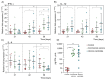
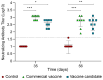
Multivalent live-attenuated or inactivated vaccines are often used to control the bovine viral diarrhea disease (BVD). Still, they retain inherent disadvantages and do not provide the expected protection. This study developed a new vaccine prototype, including the external segment of the E2 viral protein from five different subgenotypes selected after a massive screening. The E2 proteins of every subgenotype (1aE2, 1bE2, 1cE2, 1dE2, and 1eE2) were produced in mammalian cells and purified by IMAC. An equimolar mixture of E2 proteins formulated in an oil-in-water adjuvant made up the vaccine candidate, inducing a high humoral response at 50, 100, and 150 µg doses in sheep. A similar immune response was observed in bovines at 50 µg. The cellular response showed a significant increase in the transcript levels of relevant Th1 cytokines, while those corresponding to the Th2 cytokine IL-4 and the negative control were similar. High levels of neutralizing antibodies against the subgenotype BVDV1a demonstrated the effectiveness of our vaccine candidate, similar to that observed in the sera of animals vaccinated with the commercial vaccine. These results suggest that our vaccine prototype could become an effective recombinant vaccine against the BVD.
Keywords: BVDV subgenotypes; E2 glycoprotein; bovine viral diarrhea virus; subunit vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Goyal S.M., Ridpath J.F. Bovine Viral Diarrhea Virus: Diagnosis, Management and Control. Blackwell Publishing Professional; Ames, IA, USA: 2005.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

