Effects of Human Neural Stem Cells Overexpressing Neuroligin and Neurexin in a Spinal Cord Injury Model
- PMID: 39201431
- PMCID: PMC11354780
- DOI: 10.3390/ijms25168744
Effects of Human Neural Stem Cells Overexpressing Neuroligin and Neurexin in a Spinal Cord Injury Model
Abstract
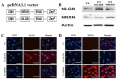
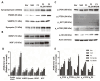
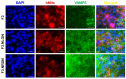
Recent studies have highlighted the therapeutic potential of stem cells for various diseases. However, unlike other tissues, brain tissue has a specific structure, consisting of synapses. These synapses not only transmit but also process and refine information. Therefore, synaptic regeneration plays a key role in therapy of neurodegenerative disorders. Neurexins (NRXNs) and neuroligins (NLGNs) are synaptic cell adhesion molecules that connect pre- and postsynaptic neurons at synapses, mediate trans-synaptic signaling, and shape neural network properties by specifying synaptic functions. In this study, we investigated the synaptic regeneration effect of human neural stem cells (NSCs) overexpressing NRXNs (F3.NRXN) and NLGNs (F3.NLGN) in a spinal cord injury model. Overexpression of NRXNs and NLGNs in the neural stem cells upregulated the expression of synaptophysin, PSD95, VAMP2, and synapsin, which are synaptic markers. The BMS scores indicated that the transplantation of F3.NRXN and F3.NLGN enhanced the recovery of locomotor function in adult rodents following spinal cord injury. Transplanted F3.NRXN and F3.NLGN differentiated into neurons and formed a synapse with the host cells in the spinal cord injury mouse model. In addition, F3.NRXN and F3.NLGN cells restored growth factors (GFs) and neurotrophic factors (NFs) and induced the proliferation of host cells. This study suggested that NSCs overexpressing NRXNs and NLGNs could be candidates for cell therapy in spinal cord injuries by facilitating synaptic regeneration.
Keywords: NLGN; NRXN; spinal cord injury; stem cells; synaptic markers; synaptic regeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

