A Newly Developed Anti-L1CAM Monoclonal Antibody Targets Small Cell Lung Carcinoma Cells
- PMID: 39201435
- PMCID: PMC11354272
- DOI: 10.3390/ijms25168748
A Newly Developed Anti-L1CAM Monoclonal Antibody Targets Small Cell Lung Carcinoma Cells
Abstract
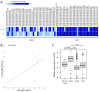
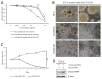
Few effective treatments are available for small cell lung cancer (SCLC), indicating the need to explore new therapeutic options. Here, we focus on an antibody-drug conjugate (ADC) targeting the L1 cell adhesion molecule (L1CAM). Several publicly available databases reveal that (1) L1CAM is expressed at higher levels in SCLC cell lines and tissues than in those of lung adenocarcinoma and (2) the expression levels of L1CAM are slightly higher in SCLC tissues than in adjacent normal tissues. We conducted a series of in vitro experiments using an anti-L1CAM monoclonal antibody (termed HSL175, developed in-house) and the recombinant protein DT3C, which consists of diphtheria toxin lacking the receptor-binding domain but containing the C1, C2, and C3 domains of streptococcal protein G. Our HSL175-DT3C conjugates theoretically kill cells only when the conjugates are internalized by the target (L1CAM-positive) cells through antigen-antibody interaction. The conjugates (an ADC analog) were effective against two SCLC-N (NEUROD1 dominant) cell lines, Lu-135 and STC-1, resulting in decreased viability. In addition, L1CAM silencing rendered the two cell lines resistant to HSL175-DT3C conjugates. These findings suggest that an ADC consisting of a humanized monoclonal antibody based on HSL175 and a potent anticancer drug would be effective against SCLC-N cells.
Keywords: ASCL1; L1 cell adhesion molecule (L1CAM); NEUROD1; antibody–drug conjugate (ADC); small cell lung cancer (SCLC).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Yamaguchi M., Hirai S., Sumi T., Tanaka Y., Tada M., Nishii Y., Hasegawa T., Uchida H., Yamada G., Watanabe A., et al. Angiotensin-converting enzyme 2 is a potential therapeutic target for EGFR -mutant lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2017;487:613–618. doi: 10.1016/j.bbrc.2017.04.102. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

