Impact of Disease Severity and Disease-Modifying Therapies on Myostatin Levels in SMA Patients
- PMID: 39201450
- PMCID: PMC11354404
- DOI: 10.3390/ijms25168763
Impact of Disease Severity and Disease-Modifying Therapies on Myostatin Levels in SMA Patients
Abstract
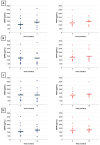
Clinical trials with treatments inhibiting myostatin pathways to increase muscle mass are currently ongoing in spinal muscular atrophy. Given evidence of potential myostatin pathway downregulation in Spinal Muscular Atrophy (SMA), restoring sufficient myostatin levels using disease-modifying treatments (DMTs) might arguably be necessary prior to considering myostatin inhibitors as an add-on treatment. This retrospective study assessed pre-treatment myostatin and follistatin levels' correlation with disease severity and explored their alteration by disease-modifying treatment in SMA. We retrospectively collected clinical characteristics, motor scores, and mysotatin and follistatin levels between 2018 and 2020 in 25 Belgian patients with SMA (SMA1 (n = 13), SMA2 (n = 6), SMA 3 (n = 6)) and treated by nusinersen. Data were collected prior to treatment and after 2, 6, 10, 18, and 30 months of treatment. Myostatin levels correlated with patients' age, weight, SMA type, and motor function before treatment initiation. After treatment, we observed correlations between myostatin levels and some motor function scores (i.e., MFM32, HFMSE, 6MWT), but no major effect of nusinersen on myostatin or follistatin levels over time. In conclusion, further research is needed to determine if DMTs can impact myostatin and follistatin levels in SMA, and how this could potentially influence patient selection for ongoing myostatin inhibitor trials.
Keywords: FSTN; GDF8; clinical trials; disease-modifying therapies; follisatin; myostatin; nusinersen; spinal muscular atrophy.
Conflict of interest statement
V.M. and J.D. are named inventors of a patent entitled “Method Relating to Myostatin Pathway Inhibition” that has been filled by UCL (PCT/GB2018/050619). L.S. gave consultancy and/or took part in the board of Biogen, Novartis, Roche, Scholar Rock, BioHaven, and Zentech.
Figures



References
-
- Finkel R.S., McDermott M.P., Kaufmann P., Darras B.T., Chung W.K., Sproule D.M., Kang P.B., Foley A.R., Yang M.L., Martens W.B. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83:810–817. doi: 10.1212/WNL.0000000000000741. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

