Hepatic Amyloid Beta-42-Metabolizing Proteins in Liver Steatosis and Metabolic Dysfunction-Associated Steatohepatitis
- PMID: 39201455
- PMCID: PMC11354580
- DOI: 10.3390/ijms25168768
Hepatic Amyloid Beta-42-Metabolizing Proteins in Liver Steatosis and Metabolic Dysfunction-Associated Steatohepatitis
Abstract
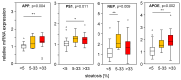
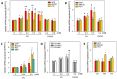
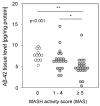
Amyloid beta (Aβ) plays a major role in the pathogenesis of Alzheimer's disease and, more recently, has been shown to protect against liver fibrosis. Therefore, we studied Aβ-42 levels and the expression of genes involved in the generation, degradation, and transport of Aβ proteins in liver samples from patients at different stages of metabolic dysfunction-associated liver disease (MASLD) and under steatotic conditions in vitro/in vivo. Amyloid precursor protein (APP), key Aβ-metabolizing proteins, and Aβ-42 were analyzed using RT-PCR, Western blotting, Luminex analysis in steatotic in vitro and fatty liver mouse models, and TaqMan qRT-PCR analysis in hepatic samples from patients with MASLD. Hepatocytes loaded with palmitic acid induced APP, presenilin, and neprilysin (NEP) expression, which was reversed by oleic acid. Increased APP and NEP, decreased BACE1, and unchanged Aβ-42 protein levels were found in the steatotic mouse liver compared to the normal liver. Aβ-42 concentrations were low in MASLD samples of patients with moderate to severe fibrosis compared to the livers of patients with mild or no MASLD. Consistent with the reduced Aβ-42 levels, the mRNA expression of proteins involved in APP degradation (ADAM9/10/17, BACE2) and Aβ-42 cleavage (MMP2/7/9, ACE) was increased. In the steatotic liver, the expression of APP- and Aβ-metabolizing proteins is increased, most likely related to oxidative stress, but does not affect hepatic Aβ-42 levels. Consistent with our previous findings, low Aβ-42 levels in patients with liver fibrosis appear to be caused by the reduced production and enhanced non-amyloidogenic processing of APP.
Keywords: MASH; MASLD; NAFLD; NASH; amyloid; fatty acids; fibrosis; oxidative stress; steatosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

